
EBK ORGANIC CHEMISTRY
6th Edition
ISBN: 9781260475685
Author: SMITH
Publisher: MCGRAW-HILL HIGHER EDUCATION
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 75P
Anacin is an over-the-counter pain reliever that contains aspirin and caffeine. Answer the following
questions about each compound.
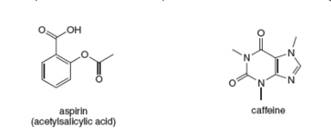
a. What is the molecular formula?
b. How many lone pairs are present on heteroatoms?
c. Label the hybridisation state of each carbon.
d. Draw three additional resonance structures.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
None
None
4. Draw and label all possible isomers for [M(py)3(DMSO)2(CI)] (py = pyridine, DMSO
dimethylsulfoxide).
Chapter 1 Solutions
EBK ORGANIC CHEMISTRY
Ch. 1.1 - While the most common isotope of nitrogen has a...Ch. 1.2 - Label each bond in the following compounds as...Ch. 1.3 - Draw a valid Lewis structure for each species. a....Ch. 1.3 - Prob. 9PCh. 1.4 - Draw Lewis structures for each molecular formula....Ch. 1.6 - Classify each pair of compounds as isomers or...Ch. 1.6 - Prob. 12PCh. 1.6 - Prob. 13PCh. 1.6 - Prob. 14PCh. 1.6 - Prob. 16P
Ch. 1.6 - Prob. 17PCh. 1.7 - Prob. 18PCh. 1.7 - Prob. 19PCh. 1.7 - Using the principles of VSEPR theory, you can...Ch. 1.8 - Convert each condensed formula to a Lewis...Ch. 1.8 - Prob. 22PCh. 1.8 - Prob. 23PCh. 1.8 - Convert each skeletal structure to a complete...Ch. 1 - Citric acid is responsible for the tartness of...Ch. 1 - Zingerone gives ginger its pungent taste. a.What...Ch. 1 - Assign formal charges to each and atom in the...Ch. 1 - Prob. 44PCh. 1 - Prob. 46PCh. 1 - Draw all possible isomers for each molecular...Ch. 1 - 1.45 Draw Lewis structures for the nine isomers...Ch. 1 - Prob. 52PCh. 1 - Prob. 53PCh. 1 - Prob. 54PCh. 1 - Consider compounds A-D, which contain both a...Ch. 1 - Draw in all the carbon and hydrogen atoms in each...Ch. 1 - 1.61 Convert each molecule into a skeletal...Ch. 1 - Prob. 65PCh. 1 - Predict the hybridization and geometry around each...Ch. 1 - Prob. 68PCh. 1 - Ketene, , is an unusual organic molecule that has...Ch. 1 - Rank the following bonds in order of increasing...Ch. 1 - Two useful organic compounds that contain Cl atoms...Ch. 1 - Use the symbols + and to indicate the polarity of...Ch. 1 - Prob. 74PCh. 1 - Anacin is an over-the-counter pain reliever that...Ch. 1 - 1.77 Stalevo is the trade name for a medication...Ch. 1 - 1.78 and are two highly reactive carbon...Ch. 1 - 1.79 The N atom in (acetamide) is hybridized,...Ch. 1 - Prob. 83PCh. 1 - Prob. 84PCh. 1 - Prob. 85PCh. 1 - Prob. 86PCh. 1 - Prob. 87PCh. 1 - Prob. 88P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The emission data in cps displayed in Table 1 is reported to two decimal places by the chemist. However, the instrument output is shown in Table 2. Table 2. Iron emission from ICP-AES Sample Blank Standard Emission, cps 579.503252562 9308340.13122 Unknown Sample 343.232365741 Did the chemist make the correct choice in how they choose to display the data up in Table 1? Choose the best explanation from the choices below. No. Since the instrument calculates 12 digits for all values, they should all be kept and not truncated. Doing so would eliminate significant information. No. Since the instrument calculates 5 decimal places for the standard, all of the values should be limited to the same number. The other decimal places are not significant for the blank and unknown sample. Yes. The way Saman made the standards was limited by the 250-mL volumetric flask. This glassware can report values to 2 decimal places, and this establishes our number of significant figures. Yes. Instrumental data…arrow_forwardSteps and explanation pleasearrow_forwardSteps and explanation to undertand concepts.arrow_forward
- Nonearrow_forward7. Draw a curved arrow mechanism for the following reaction. HO cat. HCI OH in dioxane with 4A molecular sievesarrow_forwardTry: Convert the given 3D perspective structure to Newman projection about C2 - C3 bond (C2 carbon in the front). Also, show Newman projection of other possible staggered conformers and circle the most stable conformation. Use the template shown. F H3C Br Harrow_forward
- Nonearrow_forward16. Consider the probability distribution p(x) = ax", 0 ≤ x ≤ 1 for a positive integer n. A. Derive an expression for the constant a, to normalize p(x). B. Compute the average (x) as a function of n. C. Compute σ2 = (x²) - (x)², the variance of x, as a function of n.arrow_forward451. Use the diffusion model from lecture that showed the likelihood of mixing occurring in a lattice model with eight lattice sites: Case Left Right A B C Permeable Barrier → and show that with 2V lattice sites on each side of the permeable barrier and a total of 2V white particles and 2V black particles, that perfect de-mixing (all one color on each side of the barrier) becomes increasingly unlikely as V increases.arrow_forward
- 46. Consider an ideal gas that occupies 2.50 dm³ at a pressure of 3.00 bar. If the gas is compressed isothermally at a constant external pressure so that the final volume is 0.500 dm³, calculate the smallest value Rest can have. Calculate the work involved using this value of Rext.arrow_forwardNonearrow_forward2010. Suppose that a 10 kg mass of iron at 20 C is dropped from a heigh of 100 meters. What is the kinetics energy of the mass just before it hits the ground, assuming no air resistance? What is its speed? What would be the final temperature of the mass if all the kinetic energy at impact is transformed into internal energy? The molar heat capacity of iron is Cpp = 25.1J mol-¹ K-1 and the gravitational acceleration constant is 9.8 m s¯² |arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER
Types of bonds; Author: Edspira;https://www.youtube.com/watch?v=Jj0V01Arebk;License: Standard YouTube License, CC-BY