![OWLv2 with Student Solutions Manual eBook for Masterton/Hurley's Chemistry: Principles and Reactions, 8th Edition, [Instant Access], 4 terms (24 months)](https://www.bartleby.com/isbn_cover_images/9781305863170/9781305863170_largeCoverImage.jpg)
OWLv2 with Student Solutions Manual eBook for Masterton/Hurley's Chemistry: Principles and Reactions, 8th Edition, [Instant Access], 4 terms (24 months)
8th Edition
ISBN: 9781305863170
Author: William L. Masterton; Cecile N. Hurley
Publisher: Cengage Learning US
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 72QAP
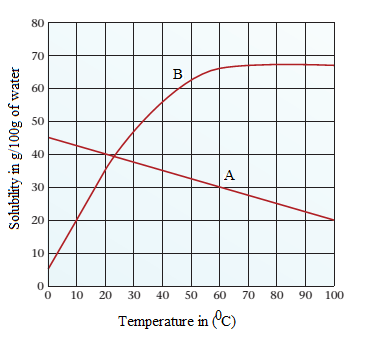
Given the following solubility curves, answer the following questions:
(a) In which of the two compounds can more solute be dissolved in the same amount of water when the temperature is decreased?
(b) At what temperature is the solubility of both compounds the same?
(c) Will an increase in temperature always increase solubility of a compound? Explain.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
. A student was given a solution of unknown molarity of Potassium Hydroxide (KOH). On the lab bench the following chemicals are kept Distilled water, Potassium Hydrogen Phthalate (KHP), HNO3, phenolphthalein and methyl or ange All required glassware is available in the lab. (a). What should be chosen as the standard solution? Justify and explain your answer by giving two properties of substances used to make standard solution. (b). What should be chosen as the indicator for the titration of nitric acid and ammonium hydroxide? Justify your answer
3. A student neutralized 25.0 cm³ of sodium hydroxide solution with 0,6
student carried out the experiment three times and obtained the following results.
Experiment 3
Experiment 1
Experiment 2
Volume of Acid
19.90
17.30
17.40
Used/ cm3
(a) The student calculated the volume of acid used by calculating the average of the volume of acid
used in Experiment 2 and Experiment 3. Explain why the first reading was not considered.
(b) Calculate the amount (moles) of 0.6 moldm³ sulfuric acid in the average titre value used to
neturalise the sodium hydroxide.
(c) How many moles of sulfuric acid react with 1 mole of sodium hydroxide?
(d) What amount (moles) of sodium hydroxide were there in the 25 cm³ sample?
Identify whether the following processes are run at constant volume and which are run at constant pressure.
(a) an acid-base titration
(b) decomposing CaCo3 by heating limestone in a crucible with a Bunsen burner
(c) the reaction between zinc metal and an aqueous solution of Cu²+ ions to form copper metal and Zn²+ ions
(d) measuring the calories in a 1-oz. serving of breakfast cereal by burning the cereal in a bomb calorimeter
Chapter 1 Solutions
OWLv2 with Student Solutions Manual eBook for Masterton/Hurley's Chemistry: Principles and Reactions, 8th Edition, [Instant Access], 4 terms (24 months)
Ch. 1 - Classify each of the following as element,...Ch. 1 - Classify each of the following as element,...Ch. 1 - Classify the following as solution or...Ch. 1 - Classify the following as solution or...Ch. 1 - Prob. 5QAPCh. 1 - Prob. 6QAPCh. 1 - Write the symbol for the following elements. (a)...Ch. 1 - Prob. 8QAPCh. 1 - Write the name of the element represented by the...Ch. 1 - Write the name of the element represented by the...
Ch. 1 - What instrument would you use to determine (a) the...Ch. 1 - What instrument would you use to (a) measure the...Ch. 1 - A glass of lukewarm milk is suggested for people...Ch. 1 - A recipe for apple pie calls for a preheated 350F...Ch. 1 - Gallium is one of the few metals that can melt at...Ch. 1 - Computers are not supposed to be in very warm...Ch. 1 - How many significant figures are in each of the...Ch. 1 - How many significant figures are there in each of...Ch. 1 - Round off the following quantities to the...Ch. 1 - Round off the following quantities to the...Ch. 1 - Prob. 21QAPCh. 1 - Prob. 22QAPCh. 1 - Which of the following statements use only exact...Ch. 1 - Which of the following statements use only exact...Ch. 1 - A basketball game at the University of...Ch. 1 - A listing of a house for sale states that there...Ch. 1 - Calculate the following to the correct number of...Ch. 1 - Perform the indicated calculations. Write your...Ch. 1 - Prob. 29QAPCh. 1 - The volume of a square pyramid is (1/3)Bh where B...Ch. 1 - Write the appropriate symbol in the blank (,,or=)....Ch. 1 - Write the appropriate symbol in the blank (,,or=)....Ch. 1 - Convert 22.3 mL to (a) liters (b) in3 (c) quartsCh. 1 - Convert 0.2156 L to (a) mL (b) in3 (c) quartsCh. 1 - The height of a horse is usually measured in...Ch. 1 - At sea, distances are measured in nautical miles...Ch. 1 - The unit of land measure in the English system is...Ch. 1 - A gasoline station in Manila, Philippines, charges...Ch. 1 - A lap in most tracks in the United States is 0.25...Ch. 1 - Cholesterol in blood is measured in milligrams of...Ch. 1 - Prob. 41QAPCh. 1 - The area of the 48 contiguous states is 3.02106...Ch. 1 - Prob. 43QAPCh. 1 - In the old pharmaceutical system of measurements,...Ch. 1 - The cup is a measure of volume widely used in...Ch. 1 - The egg whites from four large eggs occupy a...Ch. 1 - A metal slug weighing 25.17 g is added to a flask...Ch. 1 - A solid with an irregular shape and a mass of 11.3...Ch. 1 - A waterbed filled with water has the dimensions...Ch. 1 - Wire is often sold in pound spools according to...Ch. 1 - Air is 21% oxygen by volume. Oxygen has a density...Ch. 1 - The unit for density found in many density tables...Ch. 1 - Magnesium sulfate (MgSO4) has a solubility of 38.9...Ch. 1 - Potassium sulfate has a solubility of 15 g/ 100 g...Ch. 1 - Sodium bicarbonate (baking soda) is commonly use...Ch. 1 - Magnesium chloride is an important coagulant used...Ch. 1 - The solubility of lead nitrate at 100C is 140.0...Ch. 1 - Radiation exposure to human beings is usually...Ch. 1 - The following data refer to the element...Ch. 1 - A supersaturated sugar solution (650.0 g sugar in...Ch. 1 - The density of wind-packed snow is estimated to be...Ch. 1 - The dimensions of aluminum foil in a box for sale...Ch. 1 - The Kohinoor Diamond (d=3.51g/cm3) is 108 carats....Ch. 1 - A pycnometer is a device used to measure density....Ch. 1 - Prob. 65QAPCh. 1 - Label each of the properties of iodine as...Ch. 1 - How do you distinguish (a) chemical properties...Ch. 1 - What is the difference between (a) mass and...Ch. 1 - Mercury, ethyl alcohol, and lead are poured into a...Ch. 1 - How many significant figures are there in the...Ch. 1 - Consider the following solubility graph. (a) At...Ch. 1 - Given the following solubility curves, answer the...Ch. 1 - A Different civilization on a distant planet has...Ch. 1 - At what point is the temperature in F exactly...Ch. 1 - Prob. 75QAPCh. 1 - A laboratory experiment requires 12.0 g of...Ch. 1 - An average adult breathes about 8.50103 L of air...Ch. 1 - A student determines the density of a metal by...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A student weighs out a 4.80-g sample of aluminum bromide, transfers it to a 100-mL volumetric flask, adds enough water to dissolve it, and then adds water to the 100-mL mark. What is the molarity of aluminum bromide in the resulting solution?arrow_forwardUse the term soluble, insoluble, or immiscible to describe the behavior of the following pairs of substances when they are shaken together: a.25mL of water and 1g of salt the resulting mixture is clear and colorless. b.25mL of water and 1g of solid silver chloride the resulting mixture is cloudy and solid settles out. c.25mL of water and 5mL of mineral oil the resulting mixture is cloudy and gradually separates into two layers.arrow_forwardPotassium hydrogen phthalate is a solid, monoprotic acid frequently used in the laboratory as a primary standard. It has the formula KHC8H4O4. This is often written in the short-hand notation as KHP. If 25.0mL of a potassium hydroxide solution are needed to neutralize 2.26g of KHP, what is the molarity of the potassium hydroxide solution? Potassium hydrogen phthalate sometimes called potassium biphthalate, as shown on this bottle is an acid that is convenient to store and use because it is a solid.arrow_forward
- You need to make an aqueous solution of 0.123M manganese (II) acetate acetate ion, C2H3O2 for an experiment in lab, using a 250-mL volumetric flask. How much solid manganese (II) acetate should you add?arrow_forwardA solution is defined as a homogeneous mixture. Is a small sample of air a solution? Is the atmosphere a solution?arrow_forwardAn analytical procedure for finding the chloride ion concentration in a solution involves the precipitation of silver chloride: Ag++ClAgCl. What is the molarity of the chloride ion if 16.80mL of 0.629M silver nitrate the source of silver ion is needed to precipitate all of the chloride ion in a 25.00-mL sample of the unknown? Silver nitrate solution is added to an unknown chloride-ion solution, yielding a precipitate of silver chloride.arrow_forward
- ssume a highly magnified view of a solution of HCI that allows you to “see” the HCl. Draw this magnified view. If you dropped in a piece of magnesium, the magnesium would disappear, and hydrogen gas would he released. Represent this change using symbols for the elements, and write the balanced equation.arrow_forwardA student was given a 1.6240-g sample of a mixture of sodium nitrate and sodium chloride and was asked to find the percentage of each compound in the mixture. She dissolved the sample and added a solution that contained an excess of silver nitrate. The silver ion precipitated all of the chloride ion in the mixture as silver chloride. It was filtered, dried, and weighed. Its mass was 2.056g. What was the percentage of each compound in the mixture?arrow_forwardDistinguish between the solute and solvent in each of the following solutions: a saltwater [NaCl(aq)]; b sterling silver (92.5%Ag,7.5%Cu); c air about 80%N2,20%O2. On what do you base your distinctions? Sterling silver is an alloy of 92.5 silver and 7.5 another metal, usually copper.arrow_forward
- Lead poisoning has been a hazard for centuries. Some scholars believe that the decline of the Roman Empire can be traced, in part, to high levels of lead in water from containers and pipes, and from wine that was stored in leadglazed containers. If we presume that the typical Roman water supply was saturated with lead carbonate, PbCO3 (Ksp = 7.4 1014), how much lead will a Roman ingest in a year if he or she drinks 1 L/day from the container?arrow_forwardMany ionic solids dissolve in water as strong electroltyes, that is, as separated ions in solution. What properties of water facilitate this process? Would you expect ionic compounds to be soluble in elemental liquids like bromine or mercury, just as they are in water? Explain.arrow_forwardSolid Nal is added to a saturated solution of PbI₂. Assuming that the volume of the solution does not change, which statement is true about what would occur next? (A) [Pb²+] increases (B) [Pb²+] decreases (C) [Pb²+] is unchanged (D) none of the above O choice A O choice B Ochoice C O choice D Question 38 2HgO(s) + heat ⇒ 2Hg(1) + O₂(g) AH = +43.4 kcal Solid HgO, liquid Hg, and gaseous O₂ are placed in a glass bulb and are allowed to reach equilibrium at a given temperature. The mass of HgO in the bulb could be increased by (A) removing some Hg. (C) reducing the volume of the bulb. (B) removing some O₂. (D) increasing the temperature.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Solutions: Crash Course Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=9h2f1Bjr0p4;License: Standard YouTube License, CC-BY