
Chemistry Atoms First2e
2nd Edition
ISBN: 9781947172647
Author: OpenStax
Publisher: OpenStax College
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 55E
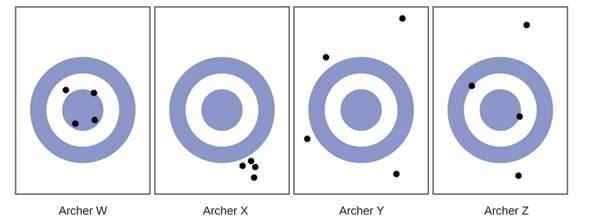
Consider the results of the archery contest shown in this figure.
- Which archer is most precise
- Which archer is most accurate
- Who is both least precise and least accurate
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
By malonic or acetylacetic synthesis, synthesize 3-methyl-4-oxopentanoic acid (indicate the formulas of the compounds).
oalmitic acid is a 16 carbon acid. In a balanced equation, the products of the sponification of tripalmitin (glyceryl tripalmitate are blank.
Write the esterification reaction mechanism of salicylic acid and acetic acid to produce aspirin (acetylsalicylic acid). Note: salicylic acid will act as the alcohol
Chapter 1 Solutions
Chemistry Atoms First2e
Ch. 1 - Explain how you could experimentally determine...Ch. 1 - Identify each of the following statements as being...Ch. 1 - Identify each of the following statements as being...Ch. 1 - Identify each of the underlined items as a part of...Ch. 1 - Identify each of the underlined items as a part of...Ch. 1 - According to one theory, the pressure of a gas...Ch. 1 - The amount of heat required to melt 2 lbs of ice...Ch. 1 - Why do we use an objects mass, rather than its...Ch. 1 - What properties distinguish solids from liquids?...Ch. 1 - How does a heterogeneous mixture differ from a...
Ch. 1 - How does a homogeneous mixture differ from a pure...Ch. 1 - How does an element differ from a compound? How...Ch. 1 - How do molecules of elements and molecules of...Ch. 1 - How does an atom differ from a molecule? In what...Ch. 1 - Many of the items you purchase are mixtures of...Ch. 1 - Classify each of the following as an element, a...Ch. 1 - Classify each of the following as an element, a...Ch. 1 - A sulfur atom and a sulfur molecule are not...Ch. 1 - How are the molecules in oxygen gas, the molecules...Ch. 1 - Why are astronauts in space said to be weightless,...Ch. 1 - Prepare a list of the principal chemicals consumed...Ch. 1 - Matter is everywhere around us. Make a list by...Ch. 1 - When elemental iron corrodes it combines with...Ch. 1 - As stated in the text, convincing examples that...Ch. 1 - Yeast converts glucose to ethanol and carbon...Ch. 1 - Classify the six underlined properties in the...Ch. 1 - Classify each of the following changes as physical...Ch. 1 - Classify each of the following changes as physical...Ch. 1 - The volume of a sample of oxygen gas changed from...Ch. 1 - A 2.0-mer volume of hydrogen gas combined with 1.0...Ch. 1 - Explain the difference between extensive...Ch. 1 - Identify the following properties as either...Ch. 1 - The density (d) of a substance is an intensive...Ch. 1 - Is one liter about an ounce, a pint, a quart, or a...Ch. 1 - Is a meter about an inch, a foot, a yard, or a...Ch. 1 - Indicate the SI base units or derived units that...Ch. 1 - Indicate the SI base units or derived units that...Ch. 1 - Give the name and symbol of the prefixes used with...Ch. 1 - Give the name of the prefix and the quantity...Ch. 1 - A large piece of jewelry has a mass of 132.6 g. A...Ch. 1 - Visit this PhET density simulation...Ch. 1 - Visit this PhET density simulation...Ch. 1 - Visit this PhET density simulation...Ch. 1 - Express each of the following numbers in...Ch. 1 - Express each of the following numbers in...Ch. 1 - Indicate whether each of the following can be...Ch. 1 - Indicate whether each of the following can be...Ch. 1 - How many significant figures are contained in each...Ch. 1 - How many significant figures are contained in each...Ch. 1 - The following quantities were reported on the...Ch. 1 - Round off each of the following numbers to two...Ch. 1 - Round off each of the following numbers to two...Ch. 1 - Perform the following calculations and report each...Ch. 1 - Perform the following calculations and report each...Ch. 1 - Consider the results of the archery contest shown...Ch. 1 - Classify the following sets of measurements as...Ch. 1 - Write conversion factors (as ratios) for the...Ch. 1 - Write conversion factors (as ratios) for the...Ch. 1 - The label on a soft drink boule gives the volume...Ch. 1 - The label on a box of cereal gives the mass of...Ch. 1 - Soccer is played with a round ball having a...Ch. 1 - A woman’s basketball has a circumference between...Ch. 1 - How many milliliters of a soft drink are contained...Ch. 1 - A barrel of oil is exactly 42 gal. How many liters...Ch. 1 - The diameter of a red blood cell is about 3104 in....Ch. 1 - The distance between the centers of the two oxygen...Ch. 1 - Is a 197-lb weight lifter light enough to compete...Ch. 1 - A very good 197-Ib weight lifter lifted 192 kg in...Ch. 1 - Many medical laboratory tests are run using 5.0 L...Ch. 1 - If an aspirin tablet Contains 325 mg aspirin, how...Ch. 1 - Use scientific (exponential) notation to express...Ch. 1 - Complete the following conversions between SI...Ch. 1 - Gasoline is sold by the liter in many countries....Ch. 1 - Milk is sold by the liter in many Countries. What...Ch. 1 - A long ton is defined as exactly 2240 lb. What is...Ch. 1 - Make the conversion indicated in each of the...Ch. 1 - Make the conversion indicated in each of the...Ch. 1 - Many chemistry conferences have held a 50-Trillion...Ch. 1 - Many chemistry conferences have held a 50-Trillion...Ch. 1 - The gas tank of a certain luxury automobile holds...Ch. 1 - As an instructor is preparing for an experiment,...Ch. 1 - To prepare for a laboratory period, a student lab...Ch. 1 - A chemistry student is 159 cm tall and weighs 45.8...Ch. 1 - In a recent Grand Prix, the winner completed the...Ch. 1 - Solve these problems about lumber dimensions. To...Ch. 1 - The mercury content of a stream was believed to be...Ch. 1 - Calculate the density of aluminum if 27.6 cm3 has...Ch. 1 - Osmium is one of the densest elements known. What...Ch. 1 - Calculate these masses. What is the mass of 6.00...Ch. 1 - Calculate these masses. What is the mass of 4.00...Ch. 1 - Calculate these volumes. What is the volume of 25...Ch. 1 - Calculate these volumes. What is the volume of...Ch. 1 - Convert the boiling temperature of gold, 2966 C,...Ch. 1 - Convert the temperature of scalding water, 54 C,...Ch. 1 - Convert the temperature of the coldest area in a...Ch. 1 - Convert the temperature of dry ice, 77 C, into...Ch. 1 - Convert the boiling temperature of liquid ammonia....Ch. 1 - The label on a pressurized can of spray...Ch. 1 - The weather in Europe was unusually warm during...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Fill in the blanks: The nose is to the mouth. The ankle is to the knee. The ring finger is to the inde...
Human Anatomy & Physiology (2nd Edition)
51. Classify each compound as ionic or molecular. If it is ionic, determine whether the metal forms only one ty...
Introductory Chemistry (6th Edition)
The following variances were calculated for two traits in a herd of hogs. (a) Calculate broad-sense (H2) and na...
Concepts of Genetics (12th Edition)
16. Explain some of the reasons why the human species has been able to expand in number and distribution to a g...
Campbell Biology: Concepts & Connections (9th Edition)
66. Astronauts use a centrifuge to simulate the acceleration of a rocket launch. The centrifuge takes 30 s to...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Endospore formation is called (a) _____. It is initiated by (b) _____. Formation of a new cell from an endospor...
Microbiology: An Introduction
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What type of interaction would you expect between the following R groups in the tertiary structure of a protein? O -CH2-CO and -CH2-CH2-CH2-CH2-NH3+ a. disulfide bonds b. salt bridges c. hydrogen bonds HO abios vist anisinoo tedt bigil s ai loistaslor sale! 10 OUT d. hydrophobic interactions e. peptide bondsarrow_forward4. True or false: This skeletal structure represents a saturated fatty acid. Ini to 0 fale) me OH faistong starrow_forwardBy malonic or acetylacetic synthesis, synthesize 5-Methyl-2-hexanone (with the formulas of the compounds).arrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' by filling in all the empty green boxes *The values are all provided in the first photo attached*arrow_forwardDraw the formula for 3-chlorobenzoic acetic anhydride.arrow_forwardBy malonic or acetylacetic synthesis, synthesize 2-methylbutanoic acid (indicate the formulas of the compounds).arrow_forward
- Obtain 2-methylbutanoic acid by malonic or acetylacetic synthesis (indicate the formulas of the compounds involved).arrow_forwardEFFICIENTS SAMPLE READINGS CONCENTRATIONS Pigiadient) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) 58 6.274 3.898 301.7 151.2 14150 5.277 3.865 348.9 254.8 B 5.136 3.639 193.7 85.9 605 4.655 3.041 308.6 199.6 05 5.135 3.664 339.5 241.4 0139 4.676 3.662 160.6 87.6 90148 5.086 3.677 337.7 242.5 0092 6.348 3.775 464.7 186.4 PART3 5.081 3.908 223.5 155.8 5.558 3.861 370.5 257.1 4.922 3.66 326.6 242.9 4.752 3.641 327.5 253.3 50 5.018 3.815 336.1 256.0 84 4.959 3.605 317.9 216.6 38 4.96 3.652 203.8 108.7 $3 5.052 3.664 329.8 239.0 17 5.043 3.767 221.9 149.7 052 5.058 3.614 331.7 236.4 5.051 4.005 211.7 152.1 62 5.047 3.637 309.6 222.7 5.298 3.977 223.4 148.7 5.38 4.24 353.7 278.2 5 5.033 4.044 334.6 268.7 995 4.706 3.621 305.6 234.4 04 4.816 3.728 340.0 262.7 16 4.828 4.496 304.3 283.2 0.011 4.993 3.865 244.7 143.6 AVERAGE STDEV COUNT 95% CI Confidence Interval (mmol/L) [Na+] (mg/100 mL) 95% Na+ Confidence Interval (mg/100 mL)arrow_forwardIf we have two compounds: acetone (CH₃COCH₃) and acetic acid (CH₃COOH), applying heat to them produces an aldol condensation of the two compounds. If this is correct, draw the formula for the final product.arrow_forward
- If we have two compounds: acetone (CH3COCH3) and acetic acid (CH3COOH); if we apply heat (A), what product(s) are obtained?arrow_forwardQUESTION: Fill out the answers to the empty green boxes attached in the image. *Ensure you all incorporate all 27 values (per column)*arrow_forwardYou need to make a buffer by dissolving benzoic acid and sodium benzoate in water. What is the mass of benzoic acid that you would weigh out, in mg, to create 50 mL of a buffer at pH = 4.7 that will change pH no more than 0.10 units with the addition of 0.001 moles of acid or base? Enter just the answer without the units (mg) - just the number will do!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Measurement and Significant Figures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=Gn97hpEkTiM;License: Standard YouTube License, CC-BY
Trigonometry: Radians & Degrees (Section 3.2); Author: Math TV with Professor V;https://www.youtube.com/watch?v=U5a9e1J_V1Y;License: Standard YouTube License, CC-BY