
ORGANIC CHEMISTRY-PRINT MULTI TERM
4th Edition
ISBN: 9781119832614
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 1, Problem 46PP
Interpretation Introduction
Interpretation: All the carbon and hydrogen atoms of the following compounds should be drawn.
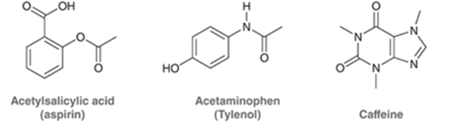
Concept Introduction: A method used to represent molecular structures of compounds is said to be bond line notation. In this notation, a line depicts a bond between two atoms and are drawn in a zigzag format. Atoms other than carbon and hydrogens are specifically depicted in this notation. It is assumed that carbon atoms are bonded to enough hydrogen atoms that are required to complete the octet.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Can you explain step by step behind what the synthetic strategy would be?
Please explain step by step in detail the reasoning behind this problem/approach/and answer. thank you!
2. Predict the product(s) that forms and explain why it forms. Assume that any necessary catalytic acid is
present.
.OH
HO
H₂N
OH
Chapter 1 Solutions
ORGANIC CHEMISTRY-PRINT MULTI TERM
Ch. 1.2 - Prob. 1LTSCh. 1.2 - Prob. 2ATSCh. 1.2 - Prob. 2LTSCh. 1.3 - Prob. 3LTSCh. 1.3 - Prob. 4PTSCh. 1.3 - Prob. 5PTSCh. 1.4 - Prob. 4LTSCh. 1.4 - Prob. 7PTSCh. 1.4 - Prob. 8PTSCh. 1.4 - Prob. 9ATS
Ch. 1.5 - Prob. 5LTSCh. 1.5 - Prob. 10PTSCh. 1.5 - Prob. 11ATSCh. 1.5 - Prob. 12ATSCh. 1.6 - Prob. 6LTSCh. 1.6 - Prob. 14ATSCh. 1.7 - Prob. 7LTSCh. 1.7 - Prob. 17ATSCh. 1.10 - Prob. 18CCCh. 1.10 - Prob. 20CCCh. 1.10 - Prob. 8LTSCh. 1.10 - Prob. 21PTSCh. 1.10 - Nemotin is a compound that was first isolated from...Ch. 1.10 - Prob. 23CCCh. 1.11 - Prob. 9LTSCh. 1.11 - Prob. 24PTSCh. 1.11 - Prob. 25PTSCh. 1.11 - Prob. 26PTSCh. 1.11 - Prob. 27ATSCh. 1.12 - Prob. 10LTSCh. 1.12 - Prob. 29ATSCh. 1.13 - Prob. 11LTSCh. 1.13 - Prob. 31ATSCh. 1 - Prob. 32PPCh. 1 - Prob. 33PPCh. 1 - Prob. 34PPCh. 1 - Prob. 35PPCh. 1 - Prob. 36PPCh. 1 - Prob. 37PPCh. 1 - Prob. 38PPCh. 1 - Prob. 39PPCh. 1 - Prob. 40PPCh. 1 - Prob. 41PPCh. 1 - Prob. 42PPCh. 1 - Prob. 44PPCh. 1 - Prob. 45PPCh. 1 - Prob. 46PPCh. 1 - Prob. 47PPCh. 1 - Prob. 48PPCh. 1 - Prob. 49PPCh. 1 - Prob. 50PPCh. 1 - Prob. 51PPCh. 1 - Prob. 52PPCh. 1 - Prob. 53PPCh. 1 - Prob. 54PPCh. 1 - Nicotine is an addictive substance found in...Ch. 1 - Prob. 56PPCh. 1 - Prob. 57PPCh. 1 - Prob. 59PPCh. 1 - Prob. 63ASPCh. 1 - Prob. 64ASPCh. 1 - Prob. 66ASPCh. 1 - Prob. 69ASPCh. 1 - Prob. 71ASPCh. 1 - Prob. 72ASPCh. 1 - Prob. 75IP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- consider the rate of the reaction below to be r. Whats the rate after each reaction? Br + NaCN CN + NaBr a. Double the concentration of alkyl bromide b. Halve the concentration of the electrophile & triple concentration of cyanide c. Halve the concentration of alkyl chloridearrow_forwardPredict the organic reactant that is involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forward
- What is the organic molecule X of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardWhat are is the organic molecule X and product Y of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardAt 300 K, in the decomposition reaction of a reactant R into products, several measurements of the concentration of R over time have been made (see table). Without using graphs, calculate the order of the reaction. t/s [R]/(mol L-1) 0 0,5 171 0,16 720 0,05 1400 0,027arrow_forward
- Predict the organic products that form in the reaction below, and draw the skeletal ("line") structures of the missing organic products. Please include all steps & drawings & explanations.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardWhat are the products of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forward
- What would happen if you added the HCI to the Grignard reagent before adding benzophenone? Draw a reaction mechanism to support your answer.arrow_forwardAt 300 K, in the decomposition reaction of a reactant R into products, several measurements of the concentration of R over time have been made (see table). Calculate the order of the reaction. t/s [R]/ (mol L-1) 0 0,5 171 0,16 720 0,05 1400 0,027arrow_forwardWrite the correct IUPAC names of the molecules in the picturearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License