
Concept explainers
a.
Interpretation: All the constitutional isomers that have molecular formulas
Concept Introduction: The molecules that possess the same molecular formula but differ in the structural arrangement of atoms in the molecule are said to be isomers of each other.
a.
Answer to Problem 32PP
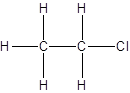
Explanation of Solution
For the first isomer structure, all two-carbon atoms are written in a straight chain and the chlorine atom is bonded to one of the carbon atoms as:
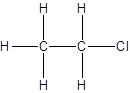
There is no other possibility for the different structural formula
b.
Interpretation: All the constitutional isomers that have molecular formulas
Concept Introduction: The molecules that possess the same molecular formula but differ in the structural arrangement of atoms in the molecule are said to be isomers of each other.
b.
Answer to Problem 32PP
Isomer I:
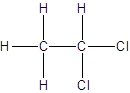
Isomer II:
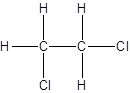
Explanation of Solution
The different structural the arrangement of atoms in the molecule
- All two-carbon atoms are written in a straight chain and two chlorine atoms are bonded to one of the carbon atoms resulting in:
- Interchanging the position of one hydrogen atom at first carbon with one chlorine atom of the second carbon atom.
Isomer I:
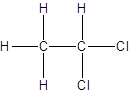
Isomer II:
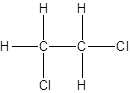
c.
Interpretation: All the constitutional isomers that have molecular formulas
Concept Introduction: The molecules that possess the same molecular formula but differ in the structural arrangements of atoms in the molecule are said to be isomers of each other.
c.
Answer to Problem 32PP
Isomer I:
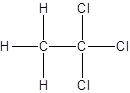
Isomer II:
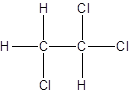
Explanation of Solution
The different structural arrangement of atoms in the molecule
- All two-carbon atoms are written in a straight chain and three chlorine atoms are bonded to one of the carbon atoms resulting in:
- Interchanging the position of one hydrogen atom at first carbon with one chlorine atom of the second carbon atom.
Isomer I:
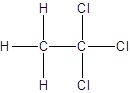
Isomer II:
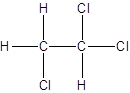
d.
Interpretation: All the constitutional isomers that have molecular formulas
Concept Introduction: The molecules that possess the same molecular formula but differ in the structural arrangements of atoms in the molecule are said to be isomers of each other.
d.
Answer to Problem 32PP
Isomer I:
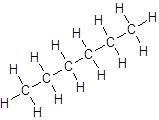
Isomer II:
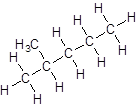
Isomer III:
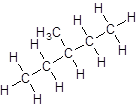
Isomer IV:
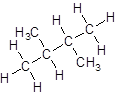
Isomer V:
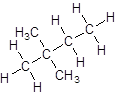
Explanation of Solution
The different structural arrangement of atoms in the molecule
- All six-carbon atoms are written in a straight chain resulting in:
- Five-carbon atoms are written in a straight chain and a methyl group is attached to the second carbon resulting in:
- Five-carbon atoms are written in a straight chain and a methyl group is attached to the third carbon resulting in:
- Four-carbon atoms are written in a straight chain and a methyl group is attached to the second and third carbon resulting in:
- Four-carbon atoms are written in a straight chain and two methyl groups are attached to the second carbon resulting in:
Isomer I:
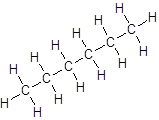
Isomer II:
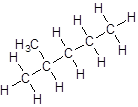
Isomer III:
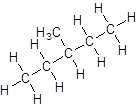
Isomer IV:
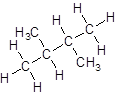
Isomer V:
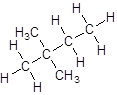
Want to see more full solutions like this?
Chapter 1 Solutions
ORGANIC CHEMISTRY-PRINT MULTI TERM
- (c) (4pts) Mechanism: heat (E1) CH3OH + 1.5pts each _E1 _ (1pt) Br CH3OH (d) (4pts) Mechanism: SN1 (1pt) (e) (3pts) 1111 I H 10 Ill!! H LDA THF (solvent) Mechanism: E2 (1pt) NC (f) Bri!!!!! CH3 NaCN (3pts) acetone Mechanism: SN2 (1pt) (SN1) -OCH3 OCH3 1.5pts each 2pts for either product 1pt if incorrect stereochemistry H Br (g) “,、 (3pts) H CH3OH +21 Mechanism: SN2 (1pt) H CH3 2pts 1pt if incorrect stereochemistry H 2pts 1pt if incorrect stereochemistryarrow_forwardA mixture of butyl acrylate and 4'-chloropropiophenone has been taken for proton NMR analysis. Based on this proton NMR, determine the relative percentage of each compound in the mixturearrow_forwardQ5: Label each chiral carbon in the following molecules as R or S. Make sure the stereocenter to which each of your R/S assignments belong is perfectly clear to the grader. (8pts) R OCH 3 CI H S 2pts for each R/S HO R H !!! I OH CI HN CI R Harrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
