
Connect Online Access 1-Semester for Organic Chemistry
6th Edition
ISBN: 9781260475609
Author: SMITH, Janice
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 43P
Assign formal charges to each
a. 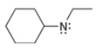 b.
b. 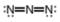 c.
c. 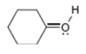 d.
d. 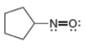
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Considering the structure on the far left as the original resonance structure, which of the subsequent resonance structures (A-D) is implausible.
Explain why you believe the resonance structure to be implausible.
The element sulphur has atomic number 16. Sulphur forms a molecular compound with chloride with a molecular formula SCl2. The atomic number of chlorine is 17.
a. Write down the electronic configuration of a sulphur atom and a chlorine atom.
b. Draw a Lewis structure of an SCl2 molecule, by showing the electrons in the outer shells of the atoms.
c. State how many bond pairs and lone pairs of electrons are arranged around the sulphur atoms in SCl2 molecule.
d. Sketch the shape and state the molecular geometry of SCl2 molecule.
e. Explain the hybridisation of the central atom in SCl2 molecule.
f.Explain the intermolecular forces exists in SCl2 molecule.
Draw a Lewis structure for SO2 that obeys the octet rule if possible and answer the following questions based on your drawing.
1. For the central sulfur atom:
- The number of lone pairs = ?
- The number of single bonds = ?
- The number of double bonds = ?
2. The central sulfur atom
a. obeys the octet rule.
b. has an incomplete octet.
c. has an expanded octet.
Chapter 1 Solutions
Connect Online Access 1-Semester for Organic Chemistry
Ch. 1.1 - While the most common isotope of nitrogen has a...Ch. 1.2 - Label each bond in the following compounds as...Ch. 1.3 - Draw a valid Lewis structure for each species. a....Ch. 1.3 - Prob. 9PCh. 1.4 - Draw Lewis structures for each molecular formula....Ch. 1.6 - Classify each pair of compounds as isomers or...Ch. 1.6 - Prob. 12PCh. 1.6 - Prob. 13PCh. 1.6 - Prob. 14PCh. 1.6 - Prob. 16P
Ch. 1.6 - Prob. 17PCh. 1.7 - Prob. 18PCh. 1.7 - Prob. 19PCh. 1.7 - Using the principles of VSEPR theory, you can...Ch. 1.8 - Convert each condensed formula to a Lewis...Ch. 1.8 - Prob. 22PCh. 1.8 - Prob. 23PCh. 1.8 - Convert each skeletal structure to a complete...Ch. 1 - Citric acid is responsible for the tartness of...Ch. 1 - Zingerone gives ginger its pungent taste. a.What...Ch. 1 - Assign formal charges to each and atom in the...Ch. 1 - Prob. 44PCh. 1 - Prob. 46PCh. 1 - Draw all possible isomers for each molecular...Ch. 1 - 1.45 Draw Lewis structures for the nine isomers...Ch. 1 - Prob. 52PCh. 1 - Prob. 53PCh. 1 - Prob. 54PCh. 1 - Consider compounds A-D, which contain both a...Ch. 1 - Draw in all the carbon and hydrogen atoms in each...Ch. 1 - 1.61 Convert each molecule into a skeletal...Ch. 1 - Prob. 65PCh. 1 - Predict the hybridization and geometry around each...Ch. 1 - Prob. 68PCh. 1 - Ketene, , is an unusual organic molecule that has...Ch. 1 - Rank the following bonds in order of increasing...Ch. 1 - Two useful organic compounds that contain Cl atoms...Ch. 1 - Use the symbols + and to indicate the polarity of...Ch. 1 - Prob. 74PCh. 1 - Anacin is an over-the-counter pain reliever that...Ch. 1 - 1.77 Stalevo is the trade name for a medication...Ch. 1 - 1.78 and are two highly reactive carbon...Ch. 1 - 1.79 The N atom in (acetamide) is hybridized,...Ch. 1 - Prob. 83PCh. 1 - Prob. 84PCh. 1 - Prob. 85PCh. 1 - Prob. 86PCh. 1 - Prob. 87PCh. 1 - Prob. 88P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Give one example from main group chemistry that illustrates each of the following descriptions: (a) Covalent ne...
General Chemistry: Atoms First
Practice Problem 1.22 Which of the following alkenes can exist as cis-trans isomers? Write their structures. Bu...
Organic Chemistry
The smallest building blocks inside your cell phone are about 1000 times smaller than the diameter of a human h...
Chemistry In Context
141. Design a device that uses as electrochemical cell to determine amount of
in a sample water Describe, in...
Chemistry: Structure and Properties (2nd Edition)
4. 38 Strontium has four naturally occurring isotopes, with mass numbers 84, 86, 87, arid 88.
a. Write the atom...
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the most polar bond in the molecule?arrow_forwardA. CHF i. Best Lewis Structure B. HNO (H is connected to one of the O's) i. Best Lewis Structure ii. Electron geometry on the C atom ii. Electron geometry on the N atom iii. Approximate bond angles about the C atom iii. Approximate bond angles around the N atom v. Draw the shape with in and out wedges (as necessary) and dipole arrows around the C atom. v. Draw the shape with in and out wedges (as necessary) and dipole arrows around the N atom. vi. Is the molecule polar or nonpolar? vi. Is the molecule polar or nonpolar?arrow_forwardDraw in all the hydrogen atoms and nonbonded electron pairs in each ion. a. b. d. ENHarrow_forward
- To answer the questions, interpret the following Lewis diagram for SO42- . 1. For the central sulfur atom: ... The number of non-bonding electrons = The number of bonding electrons = The total number of electrons = 2. The central sulfur atom fill in the blank 4 ... A. obeys the octet rule. B. has more than an octet. C. has less than an octet.arrow_forwardCarbon ring structures are common in organic chemistry. Draw a Lewis structure for each carbon ring structure, including any necessary resonance structures. a. CHg b. CH4 c. CH12 d. C,H.arrow_forwardDraw a Lewis structure for BF3 that obeys the octet rule if possible and answer the following questions based on your drawing. 1. For the central boron atom: - The number of lone pairs = ? - The number of single bonds = ? - The number of double bonds = ? 2. The central boron atom is a. obeys the octet rule. b. has an incomplete octet. c. has an expanded octet.arrow_forward
- Consider compounds A-D, which contain both a heteroatom and a double bond. N. H. A B a. For which compounds are no additional Lewis structures possible? b. When two or more Lewis structures can be drawn, draw all additional resonance structures.arrow_forwardDraw the Lewis structure for bromoethane (C,H,Br). Be certain you include any lone pairs. C. C.arrow_forwardA. Fluoromethane B. Methanol C. Chloromethane D. Water 1. Which molecule has the highest dipole moment? 2. Which molecule has the greatest bond angle relative to the electronegative atom? 3. Which molecule has the most optimal bond angle? 4. Which molecule is the most polar? 5. Which molecule contains the most electronegative atom?arrow_forward
- Now, illustrate the various bond combinations each element can form. Refer to Carbon for the example. Take note that one bond is represented by a line. Nitrogen 3 single b. 1 single, 1 double c. 1 triple Oxygen 2 single b. 1 double Carbon 4 single a. а. а. b. 2 double c. 2 single & 1 double d. 1 single & 1 triple а. a. 4 single bonds а. -C b. b. b.arrow_forwardDraw in all the hydrogen atoms and nonbonded electron pairs in each ion.arrow_forwardWith reference to cation B, label each species as an isomer, a resonance structure, or neither.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY