
Concept explainers
a. After the label fell off a bottle containing a clear liquid believed to be benzene, a chemist measured the density of the liquid to verify its identity. A 25.0-mL portion of the liquid had a mass of 21.95 g. A chemistry handbook lists the density of benzene at15 -C as
0.8787 g/ml. Is the calculated density in agreement with the tabulated value?
b An experiment requires 15 0 g of cyclohexane. whose density at 25 °C is 0.7781 g/ml. What volume of cyclohexane should be used?
c. A spherical ball of lead has a diameter of 5.0 cm. What is the mass of the sphere if lead has a
density of 11.34 g/cm3? (The volume of a sphere is (4 /3) 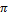
 r3, where r is the radius.)
r3, where r is the radius.)
Trending nowThis is a popular solution!

Chapter 1 Solutions
Test Prep Series for AP Chemistry for Chemistry: The Central Science 14th ed AP
- The reaction of 2-oxacyclopentanone with hydrochloric acid in water (i.e., "excess") produces which of the following carboxylic acids?arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardWhat is the name of the major product formed during the reaction between benzoyl chloride and phenol? benzyl ester O phenyl benzoate ○ cyclopentanoate ○ benzyl phenoate ○ benzenecarboxylic acidarrow_forward
- Provide the proper IUPAC or common name for the following compound. Dashes, commas, and spaces must be used correctly.arrow_forwardProvide the proper IUPAC name (only) for the following compound. Dashes, commas, and spaces must be used correctly. HO. OHarrow_forwardQuestion 2 0/1 pts Provide the proper IUPAC name only for the following compound. Dashes, commas, and spaces must be used correctly. HO CH 3 1-methyl-1-cyclohexanecarboxylic acidarrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





