
Principles of Chemistry: A Molecular Approach, Books a la Carte Edition; Modified Mastering Chemistry with Pearson eText - ValuePack Access Card - Chemistry: A Molecular Approach (3rd Edition)
3rd Edition
ISBN: 9780134172521
Author: Nivaldo J. Tro
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 36E
Read each measurement to the correct number of significant figures. Note: Laboratory glassware should always be read from the bottom of the meniscus. Digital balances normally display mass to the correct number of significant figures for that particular balance.

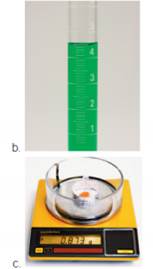
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Two separate masses of zinc were measured on a laboratory balance. The first zinc sample had a mass of 210.10 g, and the second zinc sample had a mass of 235.10 g. The two samples were combined. The volume of the combined sample was found to be 62.3 mL. Express the mass and density of the zinc sample in the correct number of significant figures.
Convert 75 miles into meters. (Use the conversion 1.0 km = 0.621 miles and use metric conversions for the rest of the conversions).
Mass (g)
Height (cm)
Diameter (cm)
Cylinder A
15.560
5.11
1.20
Cylinder B
35.536
5.90
1.30
In your calculation use the value 3.14 for π. Write your answer to the correct number of significant figures.
Calculate the volume of Cylinder A and B using the dimensions of the cylinder.
Chapter 1 Solutions
Principles of Chemistry: A Molecular Approach, Books a la Carte Edition; Modified Mastering Chemistry with Pearson eText - ValuePack Access Card - Chemistry: A Molecular Approach (3rd Edition)
Ch. 1 - For Practice 1.1 Is each change physical or...Ch. 1 - Prob. 1.2PCh. 1 - Prob. 1.3PCh. 1 - Prob. 1.3MPCh. 1 - Record the temperature on the thermometer shown...Ch. 1 - For Practice 1.5 How many significant figures are...Ch. 1 - Perform the calculations to the correct number of...Ch. 1 - Prob. 1.7PCh. 1 - Convert 9255 cm3 to gallons.
Ch. 1 - Prob. 1.9P
Ch. 1 - Prob. 1.9MPCh. 1 - Backpackers often use canisters of white gas to...Ch. 1 - Prob. 1.10MPCh. 1 - For Practice 1.11
Find the radius (r)of an...Ch. 1 - Prob. 1.12PCh. 1 - Prob. 1SAQCh. 1 - Q2. This image represent a particulate view of a...Ch. 1 - Prob. 3SAQCh. 1 - Which property of rubbing alcohol is a chemical...Ch. 1 - Prob. 5SAQCh. 1 - Prob. 6SAQCh. 1 - Q7. Determine the mass of a 1.75 L sample of a...Ch. 1 - Prob. 8SAQCh. 1 - Q9. Perform the calculation to the correct number...Ch. 1 - Prob. 10SAQCh. 1 - Prob. 11SAQCh. 1 - Prob. 12SAQCh. 1 - Prob. 13SAQCh. 1 - Prob. 14SAQCh. 1 - Prob. 15SAQCh. 1 - 1. Classify each statement as an observation, a...Ch. 1 - Classify each statement as an observation, a law...Ch. 1 - A chemist decomposes several samples of carbon...Ch. 1 - 4. When astronomers observe distant galaxies, they...Ch. 1 - 5. Classify each substance as a pure substance or...Ch. 1 - 6. Classify each substance as a pure substance or...Ch. 1 - 7. Complete the table.
Substance Pure or...Ch. 1 - 8. Complete the table.
Substance Pure or...Ch. 1 - 9. Determine whether each molecular diagram...Ch. 1 - 10. Determine whether each molecular diagram...Ch. 1 - 11. Several properties of isopropyl alcohol (also...Ch. 1 - 12. Several properties of ozone (a pollutant in...Ch. 1 - 13. Classify each property as physical or chemical...Ch. 1 - 14. Classify each property as physical or...Ch. 1 - 15. Classify each change as physical or...Ch. 1 - 16. Classify each change as physical or chemical....Ch. 1 - 17. Based on the molecular digram, classify each...Ch. 1 - Based on the molecular diagram, classify each...Ch. 1 - 19. Convert each temperature.
a. 32 °F to °C...Ch. 1 - 20. Convert each temperature.
a. 212 °F to °C...Ch. 1 - 21. The coldest temperature ever measured m the...Ch. 1 - 22. The warmest temperature ever measured in the...Ch. 1 - 23. Use the prefix multipliers to express each...Ch. 1 - 24. Use scientific notation to express each...Ch. 1 - Complete the table: a. 1245kg 1.245106 g 1.245109...Ch. 1 - 26. Express the quantity 254,998 m in each unit....Ch. 1 - 27. How many 1 cm squares would it take to...Ch. 1 - 28. How many 1 cm cubes would it take to construct...Ch. 1 - 29. A new penny has a mass of 2.49 g and a volume...Ch. 1 - 30. A titanium bicycle frame displaces 0.314 L of...Ch. 1 - 31. Glycerol is a syrupy liquid often used in...Ch. 1 - 32. A supposedly gold nugget is tested to...Ch. 1 - 33. Ethylene glycol (antifreeze) has a density of...Ch. 1 - 34. Acetone (nail polish remover) Pies a density...Ch. 1 - 35. Read each measurment to the correct number of...Ch. 1 - Read each measurement to the correct number of...Ch. 1 - 37. For each measurement, underline the zeroes...Ch. 1 - 38. For each measurement underline the zeroes that...Ch. 1 - 39. How many significant figures are in each...Ch. 1 - 40. How many significant figures are in each...Ch. 1 - 41. Which quantities are exact numbers and...Ch. 1 - 42. Indicate the number of significant figures in...Ch. 1 - 43. Round each number to four significant...Ch. 1 - 44. Round each number to three significant...Ch. 1 - 45. Perform each calculation to the correct number...Ch. 1 - 46. Perform each calculation to the correct number...Ch. 1 - 47. Perform each calculation to the correct number...Ch. 1 - Prob. 48ECh. 1 - 49. Perform each calculation to the correct number...Ch. 1 - Prob. 50ECh. 1 - 51. A flask containing 117 mL of a liquid weighs...Ch. 1 - 51. A flask containing 11.7 mL of a liquid weighs...Ch. 1 - 53. Perform each unit conversion.
a. 27.8 L to cm3...Ch. 1 - 54. Perform each unit conversion.
a. 28.9 nm to ?m...Ch. 1 - 55. Perform each unit conversion between the...Ch. 1 - 56. Perform each unit conversion between the...Ch. 1 - 57. A runner wants to run 10.0 km at a pace of 7.5...Ch. 1 - 58. A cyclist rides at an average speed of 24...Ch. 1 -
59. A European automobile has a gas mileage of...Ch. 1 -
60. A gas can holds 5.0 gallons of gasoline....Ch. 1 - 61. A modest-sized house has an area of 195m2....Ch. 1 - 62. A bedroom has a volume of 115 m3. What is its...Ch. 1 - Prob. 63ECh. 1 - Total U.S. farmland occupies 954 million acres....Ch. 1 - Prob. 65ECh. 1 - Prob. 66ECh. 1 - Prob. 67ECh. 1 - Prob. 68ECh. 1 - 69. Classify each property as intensive or...Ch. 1 - 70. At what temperatures are the readings on the...Ch. 1 - 71. Suppose you have designed a new thermometer...Ch. 1 - On a new Jekyll temperature scale water freezes at...Ch. 1 - 73. Do each calculation without using your...Ch. 1 - 74. The value of the euro was recently $l.38 U.S....Ch. 1 - Prob. 75ECh. 1 - 76. The proton has a radius of approximately cm...Ch. 1 - Prob. 77ECh. 1 - Prob. 78ECh. 1 - Prob. 79ECh. 1 - Prob. 80ECh. 1 - Prob. 81ECh. 1 - Prob. 82ECh. 1 - The Toyota Prius, a hybrid electric vehicle, has...Ch. 1 - Prob. 84ECh. 1 - Prob. 85ECh. 1 - Prob. 86ECh. 1 - A length of #8 copper wire (radius = 1.63 mm) has...Ch. 1 - Prob. 88ECh. 1 - Prob. 89ECh. 1 - Prob. 90ECh. 1 - Prob. 91ECh. 1 - Prob. 92ECh. 1 - Nanotechnology, the field of building ultra-small...Ch. 1 - Prob. 94ECh. 1 - A box contains a mixture of small copper spheres...Ch. 1 - Prob. 96ECh. 1 - Prob. 97ECh. 1 - Prob. 98ECh. 1 - A cube has an edge length of 7 cm. If it is...Ch. 1 - Prob. 100ECh. 1 - For each box, examine the blocks attached to the...Ch. 1 - Prob. 102E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Be sure to report answers with the correct number of significant figures. Length:3.55 cmWidth:2.55 cmMass:0.338 g Volume of aluminum foil (cm3):0.124 cm3 Thickness of aluminum foil (cm):arrow_forwardA piece of cardboard is 12.0 cm long and 6.3 cm wide. What is the area of this piece of cardboard in square millimeters? Enter your answer in the space provided WITHOUT units. Make sure to round your answer to the correct number of significant figures. Please give the number as a normal number, do not use scientific notation. (In example: a normal number = 4300 instead of the number being in scientific notation as = 4.3 X 103)arrow_forwardPlease don't provide handwriting solutionarrow_forward
- Use significant figures to express the inherent uncertainty in measured quantities and in calculations?arrow_forwardWhen adding or dividing measured numbers, we don’t count significant figures. Instead, we look at the precision of the measured numbers. The answer can only be as precise as the least precise measurement.arrow_forwardCalculate the density of a solid substance that causes the water level in a graduated cylinder to rise from 26.35 mL to 38.29 mL, when 15.20 g of the solid are placed in the cylinder. Use significant figures.arrow_forward
- Multiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits.arrow_forwardUsing the correct number of significant figures, calculate the perimeter of a small, rectangular mirror that is 2.814 in long, 1.429 in wide, and 0.018 in thick. Make sure to calculate the the perimeter of the front of the mirror, and not the side. Dimensions are given in inches, but the final answer should be in centimeters. Recall that 1 in = 2.54 cm exactly. (Exclude units from your answer)arrow_forwardFive silver coins are added to 454.0 mL of water. The water level rises to a volume of 545.0 mL. What is the volume of the five silver coins? Express your answer with the correct number of significant figures. Write your final answer in the space below. Don't forget to show your calculations for your answer at the end of this quiz. Please include the question number with your calculations so I know which question they are for.arrow_forward
- The weight of a metal sphere was measured to be 9.81 g. Unfortunately, the caliper that was supposed to be used in measuring the dimensions of the sphere broke. The metal that make up the sphere was identified to be an alloy of iron and chromium with a density of 7.26 g/cm3. Using the data given, calculate the radius of the metal sphere in cm. Round your answer to 2 decimal places.arrow_forwardThe dimensions of a rectangular object were obtained as shown below. Use the data to determine the area of the rectangle. Make sure your final answer is reported to the correct number of significant figures: Width 3.56 cm 1sam Length 5.425 cmarrow_forwardChange the following measurement to the appropriate SI unit. The final unit required is shown to the right of the answer box. Be sure to use correct significant figures. An automobile engine with a displacement of 4.36×102 in.3 = cm3 Change the following measurement to the appropriate SI unit. The final unit required is shown to the right of the answer box. Be sure to use correct significant figures. 221 lb man = kg Change the following measurement to the appropriate SI unit. The final unit required is shown to the right of the answer box. Be sure to use correct significant figures. 18643 ft elevation above sea level = marrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
Measurement and Significant Figures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=Gn97hpEkTiM;License: Standard YouTube License, CC-BY
Trigonometry: Radians & Degrees (Section 3.2); Author: Math TV with Professor V;https://www.youtube.com/watch?v=U5a9e1J_V1Y;License: Standard YouTube License, CC-BY