
(a)
Interpretation: For the given set of compounds the location of the partial charges that results due to inductive effect should be identified.
Concept Introduction: The nature of the bond depends on the electronegativity values of the shared electron pair of the involved atoms.
Electronegativity is the important chemical property of the elements in the periodic table. It is the tendency of the atoms to attract electrons towards it.
If the difference in electronegativity is between 0.5 and 1.7, a bond between two different electronegative atoms becomes polar. Most electronegative atoms get partial negative charge because they attract electrons from least electronegative atom towards it. The least electronegative atoms get partial positive charge because it loses electrons towards most electronegative atoms.
The process of the attraction of electrons from electron-donating atoms (less electronegative atoms) towards electron-withdrawing atoms (most electronegative atoms) is called induction. It can be represented by the following arrow:
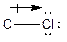
The symbol represents the direction of dipole moment change in a polar covalent bond.
Here, chlorine atom has more electronegative than carbon atom. Chlorine attracts electrons towards it. This difference in electron density is called inductive effect. It can be shown by Greek symbol delta (
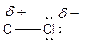
To find: The location of the partial charges in the given compound (a)
(b)
Interpretation: For the given set of compounds the location of the partial charges that results due to inductive effect should be identified.
Concept Introduction: The nature of the bond depends on the electronegativity values of the shared electron pair of the involved atoms.
Electronegativity is the important chemical property of the elements in the periodic table. It is the tendency of the atoms to attract electrons towards it.
If the difference in electronegativity is between 0.5 and 1.7, a bond between two different electronegative atoms becomes polar. Most electronegative atoms get partial negative charge because they attract electrons from least electronegative atom towards it. The least electronegative atoms get partial positive charge because it loses electrons towards most electronegative atoms.
The process of the attraction of electrons from electron-donating atoms (less electronegative atoms) towards electron-withdrawing atoms (most electronegative atoms) is called induction. It can be represented by the following arrow:
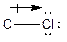
The symbol represents the direction of dipole moment change in a polar covalent bond.
Here, chlorine atom has more electronegative than carbon atom. Chlorine attracts electrons towards it. This difference in electron density is called inductive effect. It can be shown by Greek symbol delta (
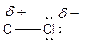
To find: The location of the partial charges in the given compound (b)
(c)
Interpretation: For the given set of compounds the location of the partial charges that results due to inductive effect should be identified.
Concept Introduction: The nature of the bond depends on the electronegativity values of the shared electron pair of the involved atoms.
Electronegativity is the important chemical property of the elements in the periodic table. It is the tendency of the atoms to attract electrons towards it.
If the difference in electronegativity is between 0.5 and 1.7, a bond between two different electronegative atoms becomes polar. Most electronegative atoms get partial negative charge because they attract electrons from least electronegative atom towards it. The least electronegative atoms get partial positive charge because it loses electrons towards most electronegative atoms.
The process of the attraction of electrons from electron-donating atoms (less electronegative atoms) towards electron-withdrawing atoms (most electronegative atoms) is called induction. It can be represented by the following arrow:
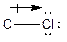
The symbol represents the direction of dipole moment change in a polar covalent bond.
Here, chlorine atom has more electronegative than carbon atom. Chlorine attracts electrons towards it. This difference in electron density is called inductive effect. It can be shown by Greek symbol delta (
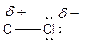
To find: The location of the partial charges in the given compound (c)
(d)
Interpretation: For the given set of compounds the location of the partial charges that results due to inductive effect should be identified.
Concept Introduction: The nature of the bond depends on the electronegativity values of the shared electron pair of the involved atoms.
Electronegativity is the important chemical property of the elements in the periodic table. It is the tendency of the atoms to attract electrons towards it.
If the difference in electronegativity is between 0.5 and 1.7, a bond between two different electronegative atoms becomes polar. Most electronegative atoms get partial negative charge because they attract electrons from least electronegative atom towards it. The least electronegative atoms get partial positive charge because it loses electrons towards most electronegative atoms.
The process of the attraction of electrons from electron-donating atoms (less electronegative atoms) towards electron-withdrawing atoms (most electronegative atoms) is called induction. It can be represented by the following arrow:
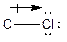
The symbol represents the direction of dipole moment change in a polar covalent bond.
Here, chlorine atom has more electronegative than carbon atom. Chlorine attracts electrons towards it. This difference in electron density is called inductive effect. It can be shown by Greek symbol delta (
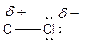
To find: The location of the partial charges in the given compound (d)
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
ORGANIC CHEMISTRY-WILEYPLUS NEXTGEN
- Show the mechanism steps to obtain the lowerenergy intermediate: *see imagearrow_forwardSoap is made by the previous reaction *see image. The main difference between one soap and another soap isthe length (number of carbons) of the carboxylic acid. However, if a soap irritates your skin, they mostlikely used too much lye.Detergents have the same chemical structure as soaps except for the functional group. Detergentshave sulfate (R-SO4H) and phosphate (R-PO4H2) functional groups. Draw the above carboxylic acidcarbon chain but as the two variants of detergents. *see imagearrow_forwardWhat are the reactions or reagents used? *see imagearrow_forward
- The two pKa values of oxalic acid are 1.25 and 3.81. Why are they not the same value? Show the protontransfer as part of your explanation. *see imagearrow_forwardасть Identify all the bonds that gauche interact with C-OMe in the most stable conformation of the above compound.arrow_forwardPredict the reactants used in the formation of the following compounds using Acid-Catalyzed dehydration reactionarrow_forward
- Can I please get help with this?arrow_forward.. Give the major organic product(s) for each of the following reactions or sequences of reactions. Show ll relevant stereochemistry [3 ONLY]. A H Br 1. NaCN 2 NaOH, H₂O, heat 3. H3O+ B. CH₂COOH 19000 1. LiAlH4 THF, heat 2 H₂O* C. CH Br 1. NaCN, acetone 2 H3O+, heat D. Br 1. Mg. ether 3. H₂O+ 2 CO₂ E. CN 1. (CH) CHMgBr, ether 2 H₂O+arrow_forwardAssign this COSY spectrumarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





