
EBK PUSHING ELECTRONS
4th Edition
ISBN: 9781285633237
Author: Weeks
Publisher: CENGAGE LEARNING - CONSIGNMENT
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 30EQ
Derive Lewis structures for the compounds below.
Azobenzene
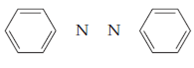
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Using iodometry I want to titrate a sodium thiosulfate solution and I use 15 mL. If I have 50 mL of a 0.90 M copper solution and KI, what will be the molarity of sodium thiosulfate?
Draw the product formed when the following pair of compounds is treated with NaOEt in ethanol.
+
i
CN
I need help with the following
Chapter 1 Solutions
EBK PUSHING ELECTRONS
Ch. 1 - 1. Hydrogen is a Group I element and each...Ch. 1 - Methanol has the molecular formula CH4O. Its...Ch. 1 - 3. The skeleton of chloromethane is...Ch. 1 - 4. Methanol’s skeleton is
Connecting all bonded...Ch. 1 - 5. The structure for chloromethane is
It...Ch. 1 - Prob. 6EQCh. 1 - 7. Dimethyl ether
No. of electrons in...Ch. 1 - Methylamine (CH5N) No. of electrons in structure...Ch. 1 - Methanethiol (CH4S) No. of electrons in structure...Ch. 1 - Methylal (C3H8O2) No. of electrons in structure...
Ch. 1 - Prob. 11EQCh. 1 - Adding electrons to the skeleton by making single...Ch. 1 - This is done by removing an unshared pair from...Ch. 1 - Prob. 14EQCh. 1 - Prob. 15EQCh. 1 - Prob. 16EQCh. 1 - The skeleton of acetyl chloride is . Write the...Ch. 1 - Three constitutional isomers exist for the formula...Ch. 1 - A number of constitutional isomers exist for the...Ch. 1 - Using the method outlined above, derive the...Ch. 1 - Prob. 21EQCh. 1 - Prob. 22EQCh. 1 - Prob. 23EQCh. 1 - Prob. 24EQCh. 1 - The skeleton of benzyldimethylamine is
The...Ch. 1 - The skeleton is benzaldoxime is The number of...Ch. 1 - Prob. 27EQCh. 1 - Derive Lewis structures for the compounds below....Ch. 1 - Prob. 29EQCh. 1 - Derive Lewis structures for the compounds below....Ch. 1 - Prob. 31EQCh. 1 - Derive Lewis structures for the compounds below....Ch. 1 - The Lewis structure of acetone is Circling the...Ch. 1 - Chloromethane has the Lewis...Ch. 1 - In the Lewis structure for chloromethane, the...Ch. 1 - Prob. 36EQCh. 1 - The oxygen atom in acetone possesses ____ unshared...Ch. 1 - Nitrobenzene has the skeleton
The number of...Ch. 1 - Prob. 39EQCh. 1 - Compute and add on the formal charges I these...Ch. 1 - Prob. 41EQCh. 1 - Prob. 42EQCh. 1 - Prob. 43EQCh. 1 - Prob. 44EQCh. 1 - Prob. 45EQCh. 1 - Prob. 46EQCh. 1 - Prob. 47EQCh. 1 - Compute and add on the formal charges in these...Ch. 1 - Prob. 49EQCh. 1 - Prob. 50EQCh. 1 - The n-propyl cation can be formed from a molecule...Ch. 1 - Prob. 52EQCh. 1 - Prob. 53EQCh. 1 - Methanol, CH3OH, is a compound in which the formal...Ch. 1 - When a proton becomes bonded to diethyl ether, by...Ch. 1 - Tetrahydrofuran has the structure
When a proton...Ch. 1 - Prob. 57EQCh. 1 - Prob. 58EQCh. 1 - The structure of pyridine is
When a proton...Ch. 1 - The carbon atom owns one electron from each of ...Ch. 1 - The n-butyl anion can be formed from When the CLi...Ch. 1 - The isobutyl anion can be formed from When the CNa...Ch. 1 - Prob. 63EQCh. 1 - Ethanol, , is a compound in which the formal...Ch. 1 - The loss of a proton attached to the oxygen atom...Ch. 1 - A very strong base can remove a proton from...Ch. 1 - Prob. 67EQCh. 1 - Prob. 68EQCh. 1 - Prob. 69EQCh. 1 - The homolysis of the OO bond in diacetyl peroxide...Ch. 1 - Prob. 71EQCh. 1 - Prob. 72EQCh. 1 - Prob. 73EQCh. 1 - Prob. 74EQCh. 1 - Prob. 75EQCh. 1 - Heterolytic cleavage of the CO bond to yield a...Ch. 1 - Prob. 77EQCh. 1 - Prob. 78EQCh. 1 - Prob. 79EQCh. 1 - Prob. 80EQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I need help with the followingarrow_forwardFor Raman spectroscopy/imaging, which statement is not true regarding its disadvantages? a) Limited spatial resolution. b) Short integration time. c) A one-dimensional technique. d) Weak signal, only 1 in 108 incident photons is Raman scattered. e) Fluorescence interference.arrow_forwardUsing a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c. (Please provide a full derivation of the equation for x from the equation for I). d) Calculate x for the 1645 cm-1 bandarrow_forward
- For CARS, which statement is not true regarding its advantages? a) Contrast signal based on vibrational characteristics, no need for fluorescent tagging. b) Stronger signals than spontaneous Raman. c) Suffers from fluorescence interference, because CARS signal is at high frequency. d) Faster, more efficient imaging for real-time analysis. e) Higher resolution than spontaneous Raman microscopy.arrow_forwardDraw the major product of the Claisen condensation reaction between two molecules of this ester. Ignore inorganic byproducts. Incorrect, 5 attempts remaining 1. NaOCH3/CH3OH 2. Acidic workup Select to Draw O Incorrect, 5 attempts remaining The total number of carbons in the parent chain is incorrect. Review the reaction conditions including starting materials and/or intermediate structures and recount the number of carbon atoms in the parent chain of your structure. OKarrow_forwardUsing a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c d) Calculate x for the 1645 cm-1 bandarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
INTRODUCTION TO MOLECULAR QUANTUM MECHANICS -Valence bond theory - 1; Author: AGK Chemistry;https://www.youtube.com/watch?v=U8kPBPqDIwM;License: Standard YouTube License, CC-BY