
Concept explainers
(a)
Interpretation:
Given line structure is to be drawn using condensed formula.
Concept introduction:
The condensed formula indicates how the atoms should be connected in a given molecule. Each non-hydrogen atom is written explicitly followed immediately by the number of hydrogen atoms that are bonded to it. Multiple bonds are not shown explicitly in the condensed structure. To arrive at a total charge of zero, each carbon should have a maximum of four bonds while each oxygen should have a maximum of two bonds and two lone pairs. A parenthesis, in the condensed formula, represents that the repetitive unit is attached to the previous carbon atom. The
Answer to Problem 1.64P
For the given line structure, the condensed structure is:
![]()
Explanation of Solution
The given line structure is:
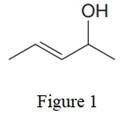
In the given line structure, there is a five carbon chain, with a carbon-carbon double bond and with a hydroxyl group. Each carbon atom is written explicitly followed immediately by the number of hydrogen atoms that are bonded to it. Multiple bonds are not shown explicitly in the condensed formula. Each carbon atom has a maximum of four bonds while each oxygen atom should have a maximum of two bonds. A parenthesis must be used for the carbon atom having three different groups attached. Thus, the hydroxyl group attached to the carbon atom must be shown in the parenthesis.
Thus, the condensed structure for the given line structure is:

The condensed structure for the given line structure is shown in Figure 2 above.
(b)
Interpretation:
Given line structure is to be drawn using condensed formula.
Concept introduction:
The condensed formula indicates how the atoms should be connected in a given molecule. Each carbon atom is written explicitly followed immediately by the number of hydrogen atoms that are bonded to it. To arrive at a total charge of zero, each carbon should have a maximum of four bonds while each oxygen should have a maximum of two bonds and two lone pairs. A parenthesis in the condensed formula represents that the repetitive unit is attached to the previous carbon atom. The
Answer to Problem 1.64P
For the given line structure, the condensed structure is:
![]()
Explanation of Solution
The given line structure is:
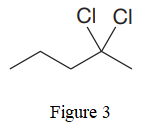
In the given line structure, there is a five carbon chain, with two chlorine atoms attached to one of the carbon atoms of the chain. Each carbon atom should have a maximum of four bonds while each oxygen atom should have a maximum of two bonds. A parenthesis must be used for the carbon atom having three different groups attached. Thus, the
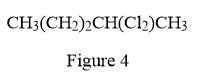
The condensed structure for the given line structure is shown in Figure 4 above.
(c)
Interpretation:
Given line structure is to be drawn using condensed formula.
Concept introduction:
The condensed formula indicates how the atoms should be connected in a given molecule. Each non-hydrogen atom is written explicitly followed immediately by the number of hydrogen atoms that are bonded to it. Multiple bonds are not shown explicitly in the condensed structure. To arrive at a total charge of zero, each carbon should have a maximum of four bonds while each oxygen should have a maximum of two bonds and two lone pairs. A parenthesis in the condensed formula represents that the repetitive unit is attached to the previous carbon atom. The
Answer to Problem 1.64P
For the given line structure, the condensed structure is:
Explanation of Solution
The given line structure is:
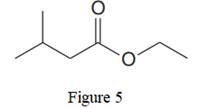
In the given line structure, there is a chain of four carbon atoms on the left side of a singly bonded oxygen. Each carbon atom is written explicitly followed immediately by the number of hydrogen atoms that are bonded to it. Multiple bonds are not shown explicitly in the condensed structure while an ethyl fragment is present at the right side of the singly bonded oxygen atom. The
Thus, the condensed structure for the given line structure is:
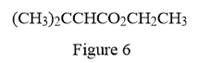
The condensed structure for the given line structure is shown in Figure 6 above.
(d)
Interpretation:
Given line structure is to be drawn using condensed formula.
Concept introduction:
The condensed formula indicates how the atoms should be connected in a given molecule. To arrive at a total charge of zero, each carbon should have a maximum of four bonds while each oxygen should have a maximum of two bonds and two lone pairs. A parenthesis in the condensed formula represents that the repetitive unit is attached to the previous carbon atom. Rings are generally now shown in their condensed formulas, but they are commonly shown in their partially condensed form. Multiple bonds in the ring and outside the ring are shows as they are. Parentheses are also used to clarify when two or three groups are attached to the same carbon atom.
Answer to Problem 1.64P
For the given line structure, the condensed structure is:
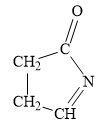
Explanation of Solution
The given line structure is:
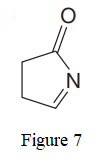
In the given line structure, a five membered ring containing a nitrogen atom is present. While writing a condensed formula, a ring is shown in its partial condensed formula. One of the carbon atoms in the ring forms a double bond with the oxygen atom which is shown as is.
Thus, the condensed structure for the given line structure is:
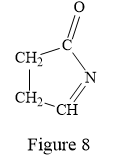
The condensed structure for the given line structure is shown in Figure 8 above.
(e)
Interpretation:
Given line structure is to be drawn using condensed formula.
Concept introduction:
The condensed formula indicates how the atoms should be connected in a given molecule. Each non-hydrogen atom is written explicitly, followed immediately by the number of hydrogen atoms that are bonded to it. Multiple bonds are not shown explicitly in the condensed structure. To arrive at a total charge of zero, each carbon should have a maximum of four bonds while each oxygen should have a maximum of two bonds and two lone pairs. A parenthesis in the condensed formula represents that the repetitive unit is attached to the previous carbon atom. Rings are generally shown in their condensed formulas, but they are commonly shown in their partially condensed form. Parentheses are also used to clarify when two or three groups are attached to the same carbon atom.
Answer to Problem 1.64P
For the given line structure, the condensed structure is:
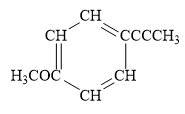
Explanation of Solution
The given line structure is:
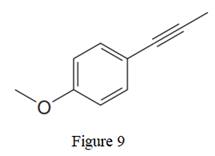
In the above line structure, the disubstituted benzene ring is present. One substituent of the benzene ring is a three carbon chain with an internal triple bond. The other substituent is a methoxy group,
Thus, the condensed structure for the given line structure is:
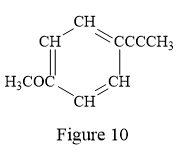
The condensed structure for the given line structure is shown in Figure 10 above.
(f)
Interpretation:
Given line structure is to be drawn using condensed formula.
Concept introduction:
The condensed formula indicates how the atoms should be connected in a given molecule. Each non-hydrogen atom is written explicitly, followed immediately by the number of hydrogen atoms that are bonded to it. To arrive at a total charge of zero, each carbon should have a maximum of four bonds while each oxygen should have a maximum of two bonds and two lone pairs. A parenthesis in the condensed formula represents that the repetitive unit is attached to the previous carbon atom. Rings are generally now shown in their condensed formulas, but they are commonly shown in their partially condensed form. Parentheses are also used to clarify when two or three groups are attached to the same carbon atom. A formal charge is shown explicitly on the atom.
Answer to Problem 1.64P
For the given line structure, the condensed structure is:
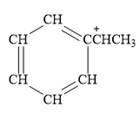
Explanation of Solution
The given line structure is:
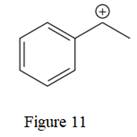
The above line structure is a structure for a cation. A carbocation is a carbon bearing a positive formal charge which is explicitly shown in the condensed formula. A six membered carbon ring with alternate double and single bonds is present and is shown as a partial condensed structure.
Thus, the condensed structure for the given line structure is:
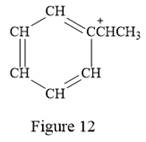
The condensed structure for the given line structure is shown in Figure 12 above.
(g)
Interpretation:
Given line structure is to be drawn using condensed formula.
Concept introduction:
The condensed formula indicates how the atoms should be connected in a given molecule. Each non-hydrogen atom is written explicitly, followed immediately by the number of hydrogen atoms that are bonded to it. To arrive at a total charge of zero, each carbon should have a maximum of four bonds while each oxygen should have a maximum of two bonds and two lone pairs. A parenthesis in the condensed formula represents that the repetitive unit is attached to the previous carbon atom. Rings are generally now shown in their condensed formulas, but they are commonly shown in their partially condensed form. Parentheses are also used to clarify when two or three groups are attached to the same carbon atom. A formal charge is shown explicitly on the atom.
Answer to Problem 1.64P
For the given line structure, the condensed structure is:
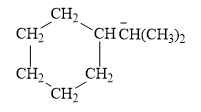
Explanation of Solution
The given line structure is:
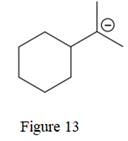
The above line structure is a structure for an anion. A six membered carbon ring with single bonds is present. Each carbon atom is written explicitly followed immediately by the number of hydrogen atoms that are bonded to it. The negative charge on the carbon atom is shown explicitly. The two methyl groups attached to the carbon bearing a negative charge are shown in the parenthesis.
Thus, the condensed structure for the given line structure is:
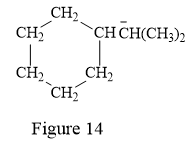
The condensed structure for the given line structure is shown in Figure 14 above.
(h)
Interpretation:
Given line structure is to be drawn using condensed formula.
Concept introduction:
The condensed formula indicates how the atoms should be connected in a given molecule. Each non-hydrogen atom is written explicitly, followed immediately by the number of hydrogen atoms that are bonded to it. To arrive at a total charge of zero, each carbon should have a maximum of four bonds while each oxygen should have a maximum of two bonds and two lone pairs. A parenthesis in the condensed formula represents that the repetitive unit is attached to the previous carbon atom. Rings are generally now shown in their condensed formulas, but they are commonly shown in their partially condensed form. Parentheses are also used to clarify when two or three groups are attached to the same carbon atom. A formal charge is shown explicitly on the atom.
Answer to Problem 1.64P
For the given line structure, the condensed structure is:
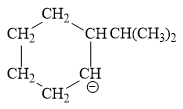
Explanation of Solution
The given line structure is:
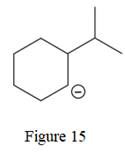
The above line structure is a six membered ring with a negative charge on one of the carbon atoms of the ring. The carbon bearing a negative charge is shown as is. The ring is monosubstituted with an isopropyl group. The two methyl groups in the isopropyl fragments is shown with the help of parenthesis.
Thus, the condensed structure for the given line structure is:
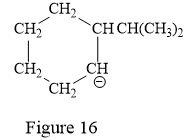
The condensed structure for the given line structure is shown in Figure 16 above.
(i)
Interpretation:
Given line structure is to be drawn using condensed formula.
Concept introduction:
The condensed formula indicates how the atoms should be connected in a given molecule. To arrive at a total charge of zero, each carbon should have a maximum of four bonds while each oxygen should have a maximum of two bonds and two lone pairs. A parenthesis in the condensed formula represents that the repetitive unit is attached to the previous carbon atom. The
Answer to Problem 1.64P
For the given line structure, the condensed structure is:
![]()
Explanation of Solution
The given line structure is:
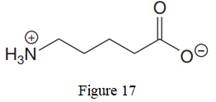
The above line structure is a chain of five carbon atoms. One end of the chain has carboxylate ion, in which the oxygen carries a negative charge. The other end of the chain has a nitrogen atom with three hydrogen atoms directly attached to it and carrying a positive charge. Formal charges on atoms are shown explicitly.
Thus, the condensed structure for the given line structure is:
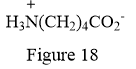
The condensed structure for the given line structure is shown in Figure 18 above.
Want to see more full solutions like this?
Chapter 1 Solutions
Get Ready for Organic Chemistry
- 1. Part 1: Naming Organic Compounds он H₁C-C-CH3 CH3 Br CI CI 2. Br-CH-CH-CH₂ H₂C-CH-C= -CH-CH2-CH3 3. HC-CH-CH-C-OH 5. H₂C-CH-CH₂-OH 7. OH 4. CH CH₂-CH₂ 6. сно CH-CH-CH-CH₂-CH₂ H₁₂C-CH-CH-CH-CH₁₂-CH₁₂ 8. OHarrow_forward11 Organic Chemistry Organic Nomenclature Practice Name/Functional Group n-butane Formula Structural Formula (1) C4tt10 H3C C- (2) CH3CH2CH2 CH 3 H₂ -CH3 Н2 name & functional group (1) and (2) OH H₁₂C Н2 name only (1) and (2) name only (1) and (2) H₁C - = - CH₂ Н2 HC=C-C CH3arrow_forwardUnder aqueous basic conditions, nitriles will react to form a neutral organic intermediate 1 that has an N atom in it first, and then they will continue to react to form the final product 2: NC H₂O он- H₂O 1 2 OH Draw the missing intermediate 1 and the final product 2 in the box below. You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forward
- Assign these COSY Spectrumarrow_forwardAssign these C-NMR and H-NMR Spectrumarrow_forwardPredict the product of this organic reaction: IZ + HO i P+H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. No Answer Click and drag to start drawing a structure. ☐ :arrow_forward
- Predict the products of this organic reaction: 0 O ----- A + KOH ? CH3-CH2-C-O-CH2-C-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. X ⑤ èarrow_forwardPredict the products of this organic reaction: O CH3 + H2O + HCI A A? CH3-CH2-C-N-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forwardWhat is the missing reactant in this organic reaction? R+ HO-C-CH2-CH3 0= CH3 CH3 —CH, C−NH—CH CH3 + H₂O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. No Answer Click anywhere to draw the first atom of your structure. €arrow_forward
- 个 CHEM&131 9267 - $25 - Intro to Mail - Hutchison, Allison (Student x Aktiv Learnin https://app.aktiv.com Draw the product of the reaction shown below. Ignore inorganic byproducts. + Na2Cr2O7 Acetone, H2SO4 Type here to search Dryng OH W Prarrow_forwardPredict the products of this organic reaction: OH + NaOH A? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click and drag to start drawing a structure. ✓ Sarrow_forwardPredict the products of this organic reaction: CH3-C-O-CH2-CH2-C-CH3 + H₂O ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. :☐ darrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

