
Concept explainers
(a)
Interpretation:
The bonding and anti-bonding combinations
Concept introduction:
The linear combination of atomic orbital (LCAO) states that two atomic orbitals combine together to form a new orbital which is known as bonding molecular orbital.
The molecular orbital theory also states that two atoms combines together to form a molecule. During the formation of a molecule, the electrons are shared between two atoms to form a
Answer to Problem 1.47AP
The bonding and anti-bonding combinations
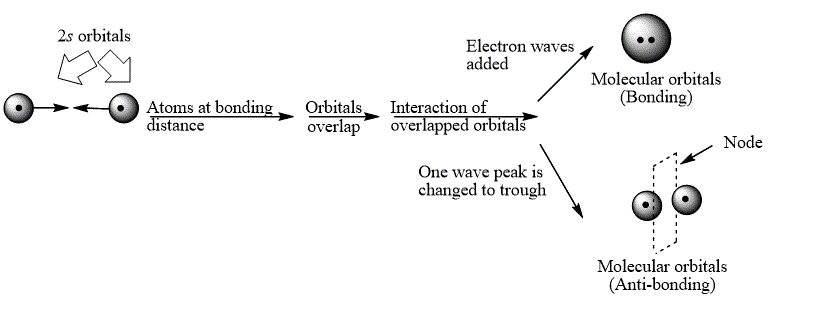
Explanation of Solution
The bond electrons which on addition form a molecular orbital are known as bonding electrons.
The bond electrons which on subtraction form a molecular orbital are known as anti-bonding electrons.
The bonding and anti-bonding combinations for the interaction of two

Figure 1
The bonding and anti-bonding molecular orbital are obtained by the overlapping of two
The bonding and anti-bonding combinations
(b)
Interpretation:
The bonding and anti-bonding combinations
Concept introduction:
The linear combination of atomic orbital (LCAO) states that two atomic orbitals combine together to form a new orbital which is known as bonding molecular orbital.
The molecular orbital theory also states that two atoms combines together to form a molecule. During the formation of a molecule, the electrons are shared between two atoms to form a chemical bond.
Answer to Problem 1.47AP
The bonding and anti-bonding combinations
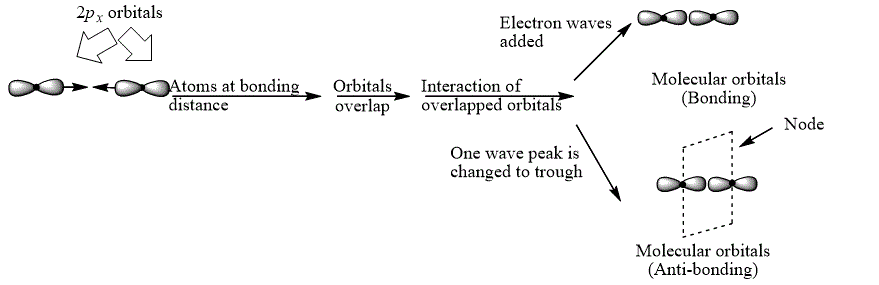
Explanation of Solution
The bond electrons which on addition form a molecular orbital are known as bonding electrons.
The bond electrons which on subtraction form a molecular orbital are known as anti-bonding electrons.
The bonding and anti-bonding combinations for the interaction of two
 Figure 2
Figure 2
The bonding and anti-bonding molecular orbital are obtained by the overlapping of two
The bonding and anti-bonding combinations
(c)
Interpretation:
The bonding and anti-bonding combinations
Concept introduction:
The linear combination of atomic orbital (LCAO) states that two atomic orbitals combine together to form a new orbital which is known as bonding molecular orbital.
The molecular orbital theory also states that two atoms combines together to form a molecule. During the formation of a molecule, the electrons are shared between two atoms to form a chemical bond.
Answer to Problem 1.47AP
The bonding and anti-bonding combinations
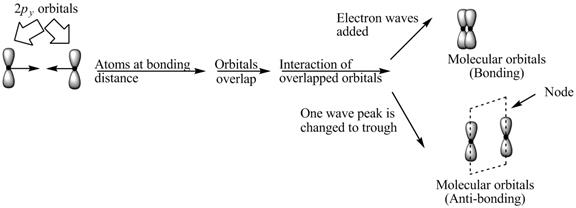
Explanation of Solution
The bond electrons which on addition form a molecular orbital are known as bonding electrons.
The bond electrons which on subtraction form a molecular orbital are known as anti-bonding electrons.
The bonding and anti-bonding combinations for the interaction of two
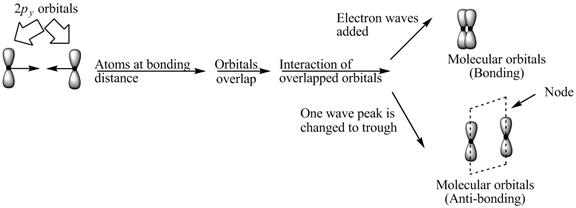
Figure 3
The bonding and anti-bonding molecular orbital are obtained by the overlapping of two
The bonding and anti-bonding combinations
(d)
Interpretation:
The bonding and anti-bonding combinations
Concept introduction:
The linear combination of atomic orbital (LCAO) states that two atomic orbitals combine together to form a new orbital which is known as bonding molecular orbital.
The molecular orbital theory also states that two atoms combines together to form a molecule. During the formation of a molecule, the electrons are shared between two atoms to form a chemical bond.
Answer to Problem 1.47AP
The bonding and anti-bonding combinations
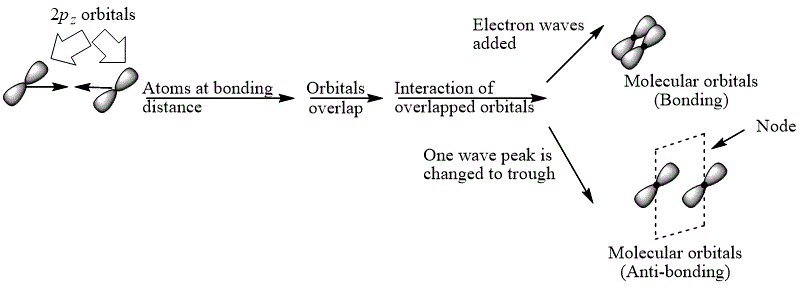
Explanation of Solution
The bond electrons which on addition form a molecular orbital are known as bonding electrons.
The bond electrons which on subtraction form a molecular orbital are known as anti-bonding electrons.
The bonding and anti-bonding combinations for the interaction of two

Figure 4
The bonding and anti-bonding molecular orbital are obtained by the overlapping of two
The bonding and anti-bonding combinations
(e)
Interpretation:
The orbital interaction energy diagram showing the energy levels of the atomic orbitals along with the energies of the given MOs is to be drawn.
Concept introduction:
The linear combination of atomic orbital (LCAO) states that two atomic orbitals combine together to form a new orbital which is known as bonding molecular orbital.
The molecular orbital theory also states that two atoms combines together to form a molecule. During the formation of a molecule, the electrons are shared between two atoms to form a chemical bond.
Answer to Problem 1.47AP
The orbital interaction energy diagram showing the energy levels of the atomic orbitals along with the energies of the given MOs is represented below.
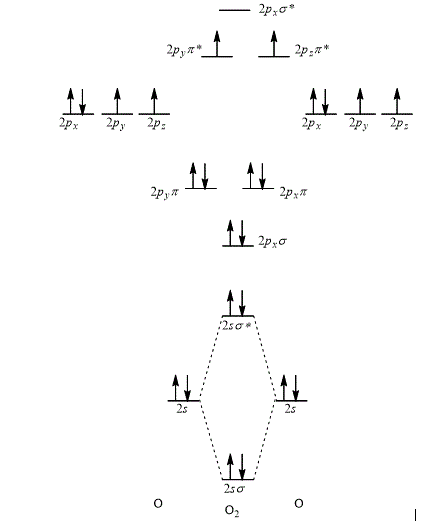
Explanation of Solution
According to the rules for filling the electrons in the molecular orbital, first of all the lower energy molecular orbital is filled followed by the filling of increasing energy order of the molecular orbital.
Thus, the orbital interaction energy diagram showing the energy levels of the atomic orbitals along with the energies of the given MOs of oxygen atom is shown as,

Figure 5
The energy of sigma,
The orbital interaction energy diagram showing the energy level of the atomic orbitals along with the energies of the given MOs is shown Figure 5.
(f)
Interpretation:
The reason corresponding to the fact that liquid
Concept introduction:
The atoms which contain no unpaired electron in their orbital and have total spin equals to zero are known as diamagnetic atoms. The atoms which contain one or more unpaired electron in their orbital are known as paramagnetic atoms.
Answer to Problem 1.47AP
The liquid
Explanation of Solution
In oxygen molecule, two unpaired electrons are present in the anti-bonding molecular orbital. Thus, oxygen molecule is paramagnetic in nature due to which it gets attracted towards the magnetic field.
In comparison to liquid oxygen, the movement of the oxygen gas molecules is faster. Therefore, liquid oxygen molecules are not attracted by the magnetic field.
Hence, liquid
The reason corresponding to the fact that liquid
(g)
Interpretation:
The Lewis structure that best describes the covalent bond(s) in
Concept introduction:
The Lewis structure shows the connectivity between atoms by identifying the lone pairs of electrons in a compound. Lewis structures are also called Lewis dot structures. The valence electrons around an atom are shown by dots. Bonds between atoms are shown by lines and the lone pair of electrons is shown by a pair of dots.
Answer to Problem 1.47AP
The Lewis structure that best describes the covalent bond(s) in
![]()
Explanation of Solution
The given Lewis structures of
The reason corresponding to the fact that liquid
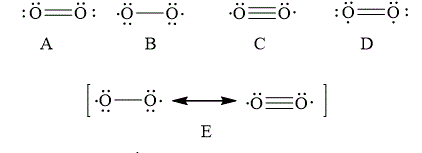
Figure 6
The total number of bonding electrons in
The total number of anti-bonding electrons in
The bond order of
Substitute the number of bonding molecular orbital and anti-bonding molecular orbital electrons in the above expression.
Thus, the bond order of oxygen molecule is
Therefore, the Lewis structures of
In option D, the Lewis structures of
In option A, the Lewis structures of
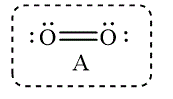
Figure 7
The Lewis structure that best describes the covalent bond(s) in
Want to see more full solutions like this?
Chapter 1 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- Can you explain how I get these here and show the steps plz?arrow_forwardGive the IUPAC name for this compound Hydrocarbon Condensed Formulas Hint C2H5 CH2CH3 expand that in all the formula Part A: (CH3)2CHCH(C2H5)CH2CH2CH3 Give the IUPAC name for this compound. Part B: CH2=C(C2H5)CH2CH2CH3 Give the IUPAC name for this compound. Part C: (CH3)2C=CHC(C2H5)=CH2 Give the IUPAC name for this compound. Part D: CH3C=CCH(C2H5)2 Give the IUPAC name for this compound. Part E: (CH3)3CC=CCH2CH=C(CH3)2arrow_forwardSelect/ Match the correct letter from the image below for the IUPAC names given below: A B C D 3 E F G H K L Part 1. 4-methylheptane For example.mmmm Answer Letter H _for part 1 Part 2. 2,4-dimethylhexane Part 3. 2,3-dimethylpentane Part 4. 2,2-dimethylhexane Part 5. 2-ethyl-1,1,3,3-tetramethylcyclopentane Part 6. 3-ethyl-2-methylpentanearrow_forward
- Can u show the process as to how to get these?arrow_forwardSketch the expected 'H NMR spectra for the following compound. Label all of the H's in the structure and the corresponding signal for the spectra you sketch. Make sure you include the integration value and the splitting pattern for each signal Indicate how many signals you would expect in the 13C NMRarrow_forwardUse IUPAC naming rules to name the following hydrocarbon compounds: CH2-CH3 | a) CH-CH-CH2-CH-CH-CH3 b) | CH2 CH3 | CH3 CH3 \ / C=C H 1 H CH2-CH3 c) d) CH=C-CH3 e) CH3-CH2-CH2-CH=CH-CH3 f) CH2=CH-CH2-CH=CH-CH3 g) CH3-CH2-C = C-CH2-CH3 h)arrow_forward
- Q5 Name the following : a. b. C. d. e.arrow_forward25. Predict the major product of the following reaction. 1 equivalent of each of the starting materials was used. H₂C CH3 CH3 H3C H3C H3C. CH2 + H3C. heat CH3 CH H.C. CH3 H.C H.C CH3 CH CH3 CH3 A B C Earrow_forwardFind chemical structures based on the below information. a) Chemical formula C6H8O Compound is aromatic plus has two 1H NMR peaks that integrated for 3 each that are singlets (it could have more peaks in the 1H NMR b) Chemical Formula: C6H100 Compounds is conjugated 'H NMR has a signal that integrates for 6 and is a doublet IR spectra has a signal at 1730 cm-1arrow_forward
- Jaslev Propose a synthesis of the following starting from benzene and any other reagents and chemicals. No mechanisms are required. Indicate the condition for each step plus the major product for each step. More than two steps are required. Step 1 Step 2 مہد Brarrow_forwardPart C: The line formula for another branched alkane is shown below. i. In the IUPAC system what is the root or base name of this compound? ii. How many alkyl substituents are attached to the longest chain? iii. Give the IUPAC name for this compound.arrow_forwardPart D: Draw the Structural Formula for 4-ethyl-2-methylhexane Part E. Draw the Structural Formula for 1-chloro-3,3-diethylpentane (Chloro = Cl)arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





