
Concept explainers
(a)
Interpretation:
Lewis structure for the given molecule is to be completed.
Concept introduction:
Lewis structures involve only valence electrons. When drawing a Lewis structure, the first step is to calculate the total number of valence electrons. For a complete Lewis structure of a molecule, the atoms must complete their normal valency by bond formation and lone pairs of electrons. Maximum number of covalent bonds formed by any neutral atom with maximum number of lone pairs is
| Atom | Number of bond | Number of lone pairs |
| C | 4 | 0 |
| H | 1 | 0 |
| O | 2 | 2 |
| N | 1 | 1 |
| F | 1 | 3 |
Answer to Problem 1.46P
The complete Lewis structure for the given molecule is
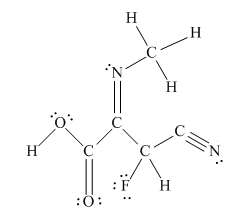
Explanation of Solution
The given structure is
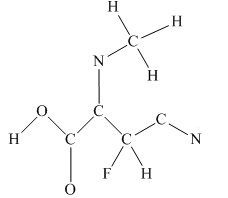
Total valence electron count for the given molecule is
The other oxygen atom has formed only one bond with carbon. This is converted to a double bond and two lone pairs are placed on the oxygen atom so that its octet is complete. A double bond is placed between C and N atom to complete the octet of carbon and a lone pair is placed in nitrogen to complete its octet.
A triple bond is placed between the other C and N to complete the octet of carbon and a lone pair is placed in the nitrogen to complete its octet.

This structure now accounts for all 54 electrons and the octet of each atom, except hydrogen, is complete. The duet for all hydrogens is complete.
The Lewis structure for the given molecule is completed from total valence electron count.
(b)
Interpretation:
Lewis structure for the given molecule is to be completed.
Concept introduction:
Lewis structures involve only valence electrons. When drawing a Lewis structure, the first step is to calculate the total number of valence electrons. For a complete Lewis structure of a molecule, every carbon atom must form four covalent bonds whereas the hydrogen atom forms one bond.
Answer to Problem 1.46P
The complete Lewis structure for the given molecule is
Explanation of Solution
The given structure is
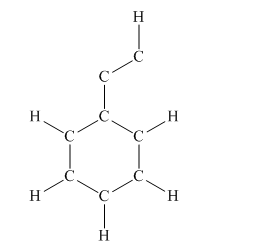
Total valence electron count for the given molecule must be

This structure now accounts for all 38 electrons and the octet of each atom, except hydrogen, is complete. The duet for all hydrogen atoms is complete.
The Lewis structure for the given molecule is completed from total valence electron count.
(c)
Interpretation:
Lewis structure for the given molecule is to be completed.
Concept introduction:
Lewis structures involve only valence electrons. When drawing a Lewis structure, the first step is to calculate the total number of valence electrons. For a complete Lewis structure of a molecule, the atoms must complete their normal valency by bond formation and lone pairs of electrons. Maximum numbers of covalent bonds formed by any neutral atom with maximum number of lone pair are
| Atom | Number of bond | Number of lone pairs |
| C | 4 | 0 |
| H | 1 | 0 |
| O | 2 | 2 |
| N | 1 | 1 |
Answer to Problem 1.46P
The complete Lewis structure for the given molecule is
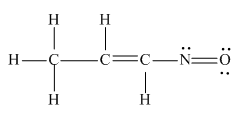
Explanation of Solution
The given structure is
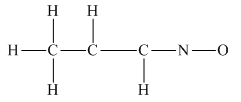
Total valence electron count for the given molecule is
The oxygen atom has formed only one bond with nitrogen. This is converted to a double bond and two lone pairs are placed on the oxygen atom so that its octet is complete. Another lone pair is placed on the nitrogen atom so that its octet is complete.
A double bond is placed between the C atoms attached to one hydrogen each. This completes the octet of both carbon atoms

This structure now accounts for all the 28 electrons, and the octet of each atom, except hydrogen, is complete. The duet for all hydrogen atoms is complete.
The Lewis structure for the given molecule is completed from total valence electron count.
Want to see more full solutions like this?
Chapter 1 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- How many chiral centers are there in the following molecule? HO 0 1 ○ 2 ♡ 4 'N'arrow_forwardThe following chemical structure represents a molecule of what molecular formula?arrow_forwardWhich region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forward
- Given the following data, determine the order of the reaction with respect to H2. H2(g) + 21Cl(g) → I2(g) + 2HCl(g) Experiment [H2] (torr) [ICI] (torr) Rate (M/s) 1 250 325 0.266 2 250 81 0.0665 3 50 325 0.266arrow_forwardWhich one of the following molecules is chiral? H- NH₂ H3C དང་།་ OH H HO H₂N HO- -H CHO -OH H HO- OH H- -H CH₂OH OHarrow_forwardThe structure of an unsaturated phospholipid is shown below. Which region of the molecule is most hydrophilic ? H₂N-CH₂ H₂C IV CH3 CH3 hydro-water philic-likes = Hydrophilic likes water ○ IV All regions are equally hydrophilic. IIIarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

