
Concept explainers
(a)
Interpretation:
Out of the two distances between two nitrogen atoms, 50 pm or 75 pm, it is to be determined which one represents higher energy.
Concept introduction:
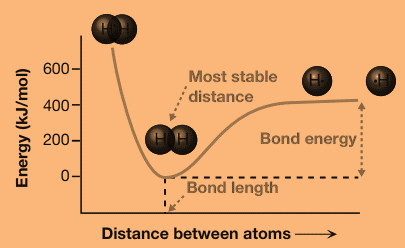
The energy of the molecule depends on the distance between the two atoms. At a certain distance, called the bond length, the energy reaches a minimum. As the distance between the two atoms increases, the energy of the molecule increases and slowly approaches a constant value as the distance increases to infinity. At distances shorter than the bond length, the energy increases very rapidly.
(b)
Interpretation:
Out of the two distances between two nitrogen atoms, 75 pm or 110 pm, it is to be determined which one represents higher energy.
Concept introduction:
The energy of the molecule depends on the distance between the two atoms. At a certain distance, called the bond length, the energy reaches a minimum. As the distance between the two atoms increases, the energy of the molecule increases and slowly approaches a constant value as the distance increases to infinity. At distances shorter than the bond length, the energy increases very rapidly.

(c)
Interpretation:
Out of the two distances between two nitrogen atoms, 110 pm or 150 pm, it is to be determined which one represents higher energy.
Concept introduction:
The energy of the molecule depends on the distance between the two atoms. At a certain distance, called the bond length, the energy reaches a minimum. As the distance between the two atoms increases, the energy of the molecule increases and slowly approaches a constant value as the distance increases to infinity. At distances shorter than the bond length, the energy increases very rapidly.

(d)
Interpretation:
Out of the two distances between two nitrogen atoms, 150 pm or 160 pm, it is to be determined which one represents higher energy.
Concept introduction:
The energy of the molecule depends on the distance between the two atoms. At a certain distance, called the bond length, the energy reaches a minimum. As the distance between the two atoms increases, the energy of the molecule increases and slowly approaches a constant value as the distance increases to infinity. At distances shorter than the bond length, the energy increases very rapidly.

Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- How many chiral centers are there in the following molecule? HO 0 1 ○ 2 ♡ 4 'N'arrow_forwardThe following chemical structure represents a molecule of what molecular formula?arrow_forwardWhich region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forward
- Given the following data, determine the order of the reaction with respect to H2. H2(g) + 21Cl(g) → I2(g) + 2HCl(g) Experiment [H2] (torr) [ICI] (torr) Rate (M/s) 1 250 325 0.266 2 250 81 0.0665 3 50 325 0.266arrow_forwardWhich one of the following molecules is chiral? H- NH₂ H3C དང་།་ OH H HO H₂N HO- -H CHO -OH H HO- OH H- -H CH₂OH OHarrow_forwardThe structure of an unsaturated phospholipid is shown below. Which region of the molecule is most hydrophilic ? H₂N-CH₂ H₂C IV CH3 CH3 hydro-water philic-likes = Hydrophilic likes water ○ IV All regions are equally hydrophilic. IIIarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

