
Interpreting Data Trans fats are a form of dietary fat that can have significant health consequences. The graph below represents data from a 2004 study that compared the amount of trans fats found in adipose tissue (body fat) of 79 heart attack patients and 167 people who did not have a heart attack. Write a one-sentence summary of the results presented in the graph.
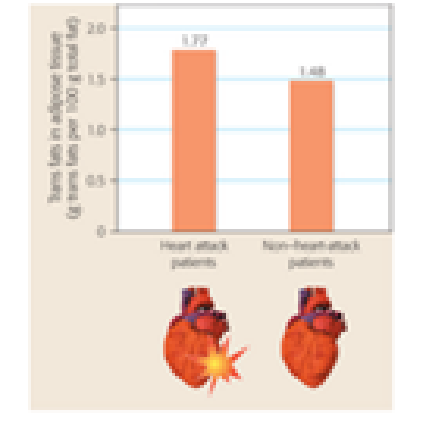
Data from: P. M. Clifton et al., Trans fatty acids in adipose tissue and the food supply are associated with myocardial infarction. Journal of Nutrition 134: 874–879 (2004).
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
Campbell Essential Biology with Physiology (5th Edition)
Additional Science Textbook Solutions
Biological Science (6th Edition)
SEELEY'S ANATOMY+PHYSIOLOGY
Physical Universe
Biology: Life on Earth with Physiology (11th Edition)
Laboratory Manual For Human Anatomy & Physiology
Cosmic Perspective Fundamentals
- 18. Watch this short youtube video about SARS CoV-2 replication. SARS-CoV-2 Life Cycle (Summer 2020) - YouTube.19. What is the name of the receptor that SARS CoV-2 uses to enter cells? Which human cells express this receptor? 20. Name a few of the proteins that the SARS CoV-2 mRNA codes for. 21. What is the role of the golgi apparatus related to SARS CoV-2arrow_forwardState the five functions of Globular Proteins, and give an example of a protein for each function.arrow_forwardDiagram of check cell under low power and high powerarrow_forward
- a couple in which the father has the a blood type and the mother has the o blood type produce an offspring with the o blood type, how does this happen? how could two functionally O parents produce an offspring that has the a blood type?arrow_forwardWhat is the opening indicated by the pointer? (leaf x.s.) stomate guard cell lenticel intercellular space none of thesearrow_forwardIdentify the indicated tissue? (stem x.s.) parenchyma collenchyma sclerenchyma ○ xylem ○ phloem none of thesearrow_forward
- Where did this structure originate from? (Salix branch root) epidermis cortex endodermis pericycle vascular cylinderarrow_forwardIdentify the indicated tissue. (Tilia stem x.s.) parenchyma collenchyma sclerenchyma xylem phloem none of thesearrow_forwardIdentify the indicated structure. (Cucurbita stem l.s.) pit lenticel stomate tendril none of thesearrow_forward
- Identify the specific cell? (Zebrina leaf peel) vessel element sieve element companion cell tracheid guard cell subsidiary cell none of thesearrow_forwardWhat type of cells flank the opening on either side? (leaf x.s.) vessel elements sieve elements companion cells tracheids guard cells none of thesearrow_forwardWhat specific cell is indicated. (Cucurbita stem I.s.) vessel element sieve element O companion cell tracheid guard cell none of thesearrow_forward
 Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage LearningNutritional Sciences: From Fundamentals to Food, ...Health & NutritionISBN:9781337486415Author:McGuirePublisher:Cengage
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage LearningNutritional Sciences: From Fundamentals to Food, ...Health & NutritionISBN:9781337486415Author:McGuirePublisher:Cengage Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning





