
EBK INTRODUCTION TO CHEMISTRY
5th Edition
ISBN: 9781260162165
Author: BAUER
Publisher: MCGRAW HILL BOOK COMPANY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 11PP
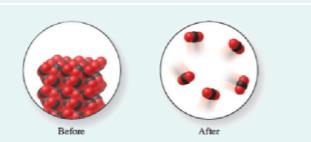
Do the following molecular level images represent a chemical change or a physical change?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Indicate which one of the following reactions most certainly results in a negative AS sys.
O1402(g) + 3NH4NO3 (s) + C10 H22(1) → 3N2(g) + 17H2O(g) + 10CO2(g)
○
CO2(aq) = CO2(g)
○ H₂O(g) = H₂O(s)
CaCO3(g) = CaO(s) + CO2(g)
O CuSO4.5H2O(s) = CuSO4(s) + 5H2O(g)
Estimate the DH°rxn of the reaction below:
H
H-C-C=C-H
H
Н
A table of bond energy
Bond
H
Bond Energy
(kJ/mol)
C-H
413
C-O
360
C=O
743
C-C
348
|C = C
612
O-H
463
H-H
436
+
H-H
->
H
H-C.
-
H
| | 1
HHH
Show work...don't give Ai generated solution
Chapter 1 Solutions
EBK INTRODUCTION TO CHEMISTRY
Ch. 1 - What characteristics distinguish different types...Ch. 1 - What are some properties of matter?Ch. 1 - What is energy and how does it differ from matter?Ch. 1 - What approaches do scientists use to answer these...Ch. 1 - Identify the non-metals in �gure 1.4. Explain...Ch. 1 - (a) Lead is a soft dull, silver-colored metal....Ch. 1 - Which of the pictures represent mixtures? Which...Ch. 1 - (a) Which of the images represents an element that...Ch. 1 - Anna and Bill see an aluminum recycling truck pass...Ch. 1 - Anna and Bill saw balloons outside the bookstore....
Ch. 1 - Solve the following problems. (a) The density of...Ch. 1 - Helium balloons rise in air. which is a mixture of...Ch. 1 - (a) The boiling point of acetylene is 28.1C. Below...Ch. 1 - Which of the following are physical properties and...Ch. 1 - Do the following molecular level images represent...Ch. 1 - Which of the two samples of argon gas is at a...Ch. 1 - Prob. 13PPCh. 1 - (a) Convert 0.0123 to scienti�c notation. (b)...Ch. 1 - Perform the following operations without using...Ch. 1 - Determine the number of signi�cant �gures in...Ch. 1 - Prob. 17PPCh. 1 - Express the answers to the following operations...Ch. 1 - Prob. 19PPCh. 1 - Round each of the following numbers to two...Ch. 1 - Convert 0.0276 kg to grams.Ch. 1 - A tablet of a typical pain reliever contains 200...Ch. 1 - The TGV POS high-speed train in France has a...Ch. 1 - Match the key terms with the following...Ch. 1 - Match the key terms with the following...Ch. 1 - Convert each of the following values to...Ch. 1 - Convert each of the following values to...Ch. 1 - Convert each of the following values from...Ch. 1 - Convert each of the following Values from...Ch. 1 - Prob. 7QPCh. 1 - For each of the following, carry out the...Ch. 1 - Determine the number of signi�cant �gures in...Ch. 1 - Determine the number of signi�cant �gures in...Ch. 1 - Express the answers to the following operations...Ch. 1 - Express the answers to the following operations...Ch. 1 - Express the answers to the following operations...Ch. 1 - Express the answers to the following operations...Ch. 1 - Express the answers to the following calculations...Ch. 1 - Express the answers to the following calculations...Ch. 1 - Round each of the following numbers to three...Ch. 1 - Round each of the following numbers to three...Ch. 1 - Carry out the following conversions. Report your...Ch. 1 - Carry out the following conversions. Report your...Ch. 1 - Carry out the following conversions. Report your...Ch. 1 - Prob. 22QPCh. 1 - Carry out the following conversions. Report your...Ch. 1 - Prob. 24QPCh. 1 - How would you classify the following items...Ch. 1 - How would you classify the following items...Ch. 1 - Which of the following are examples of matter? (a)...Ch. 1 - Which of the following are not examples of matter?...Ch. 1 - How are elements distinguished from compounds?Ch. 1 - How are homogeneous mixtures distinguished from...Ch. 1 - List characteristics of metals.Ch. 1 - List characteristics of nomnetals.Ch. 1 - Name the following elements. (a) Ti (b) Ta (c)Th...Ch. 1 - Name the following elements. (a) C (b) Ca (c) Cr...Ch. 1 - Name the following elements. (a) B (b) Ba (c) Be...Ch. 1 - Name the following elements. (a) S (b) Si (c) Se...Ch. 1 - Name the following elements. (a) N (b) Fe (c) Mn...Ch. 1 - Name the following elements. (a) Be (b) Rb (c) Ni...Ch. 1 - what are the symbols for the following elements?...Ch. 1 - What are the symbols for the following elements?...Ch. 1 - A chemical novice used the symbol It to represent...Ch. 1 - A chemical novice used the symbol SI to represent...Ch. 1 - The symbol NO was used by a student to represent...Ch. 1 - A student used the symbol CO to represent cobalt,...Ch. 1 - Classify each of the following as a pure...Ch. 1 - Classify each of the following as a pure...Ch. 1 - Elemental hydrogen normally exists as two hydrogen...Ch. 1 - Elemental chlorine normally exists as two chlorine...Ch. 1 - This image is a representation for a compound...Ch. 1 - This image represents a compound containing...Ch. 1 - Which of the images represents a mixture of an...Ch. 1 - Which of the images in question 1.51 represents a...Ch. 1 - Classify each of the following as an element or a...Ch. 1 - Prob. 54QPCh. 1 - Under normal conditions, mercury is a liquid. Draw...Ch. 1 - Under normal conditions. bromine is a liquid. Draw...Ch. 1 - What type of matter expands to �ll its container...Ch. 1 - What type of matter is composed of panicles that...Ch. 1 - Prob. 59QPCh. 1 - Identify �le physical state of each of the...Ch. 1 - What physical state is represented in this...Ch. 1 - Draw a picture of the gaseous state of the...Ch. 1 - How might you symbolically represent a homogeneous...Ch. 1 - Why does the symbol H2O(aq) make no sense?Ch. 1 - At the beginning of the chapter, Anna and Bill...Ch. 1 - At the beginning of the chapter, you were asked to...Ch. 1 - Prob. 67QPCh. 1 - A package of Swiss cheese has a mass of 0.340 kg....Ch. 1 - A grain of salt has a mass of about 1.0104g . What...Ch. 1 - If a dog has a mass of 15.2 kg, what is its mass...Ch. 1 - If you drank 1.2 L of a sports drink, what volume...Ch. 1 - If the volume of helium in a balloon is 145cm3 ,...Ch. 1 - If the length, width, and height of a box are 8.0...Ch. 1 - If a cubic box (all sides the same length) has a...Ch. 1 - A slice of cheese has a mass of 28g and a Volume...Ch. 1 - Prob. 76QPCh. 1 - If the density of a sugar solution is 1.30g/mL,...Ch. 1 - The density of a certain type of plastic is...Ch. 1 - Why do liquids have greater densities than gases?Ch. 1 - When a balloon filled with air is heated the...Ch. 1 - A piece of plastic sinks in oil but floats in...Ch. 1 - what special molecular-level feature of ice...Ch. 1 - Acetone, a component of some types of fingernail...Ch. 1 - The boiling point of liquid nitrogen is 77 K. What...Ch. 1 - What is the difference in temperature between the...Ch. 1 - If the temperature of a cup of coffee decreases...Ch. 1 - Does the boiling point of a substance depend on...Ch. 1 - Does the melting point of a substance depend on...Ch. 1 - Identify each of the following as a physical...Ch. 1 - Identify each of the following as a physical...Ch. 1 - Identify each of the following as a physical...Ch. 1 - Identify each of the following as a physical...Ch. 1 - Write a symbolic representation and a molecular...Ch. 1 - Write a symbolic representation and a...Ch. 1 - Do the changes shown in this diagram represent a...Ch. 1 - Do the Changes shown in this diagram represent a...Ch. 1 - Draw a picture that shows CH4 (shown in the...Ch. 1 - Draw a picture that shows water boiling. Does this...Ch. 1 - The image shows what happens when iodine I2 is...Ch. 1 - The picture shows natural gas CH4 burning in the...Ch. 1 - Anna and Bill saw a construction Worker welding...Ch. 1 - Bill and Anna watched students playing volleyball...Ch. 1 - Which of these two samples of carbon dioxide gas...Ch. 1 - Which of these two samples of methane gas is at a...Ch. 1 - Prob. 105QPCh. 1 - Prob. 106QPCh. 1 - Distinguish between the different types of energy...Ch. 1 - Prob. 108QPCh. 1 - Prob. 109QPCh. 1 - Prob. 110QPCh. 1 - In terms of kinetic and potential energy. Describe...Ch. 1 - In terms of kinetic and potential energy. Describe...Ch. 1 - Body mass index (BMI) is a number calculated from...Ch. 1 - Prob. 114QPCh. 1 - Prob. 115QPCh. 1 - Explain the difference between a hypothesis and a...Ch. 1 - Explain how a hypothesis is used in scientific...Ch. 1 - Classify each of the following as an observation,...Ch. 1 - Classify each of the following as an observation,...Ch. 1 - You observe coins in a fountain and propose the...Ch. 1 - You observe a piece of balsa wood floating on...Ch. 1 - Rank the following measurements in order from...Ch. 1 - The density of air in a balloon is less at high...Ch. 1 - If the temperature in a room increases from...Ch. 1 - If you have a sample of zine and a sample of...Ch. 1 - Give the symbols for potassium and phosphorus.Ch. 1 - Prob. 127QPCh. 1 - The red blood cell (RBC) Count for a normal female...Ch. 1 - Recycling facilities around the world use a...Ch. 1 - These sample of metals have the same mass. Which...Ch. 1 - The typical dose of epinephrine at a particular...Ch. 1 - About 70 million tons of paper are used per year...Ch. 1 - During a typical physical exam, blood tests to...Ch. 1 - The densities of antifreeze, corn oil, dish...Ch. 1 - The lower possible temperature is the temperature...Ch. 1 - Classify the substance in the molecular-level...Ch. 1 - Classify each of the following as a symbolic...Ch. 1 - Titanium is a strong metal with a low density that...Ch. 1 - Blood is a water-based liquid in which solids...Ch. 1 - Prob. 140QPCh. 1 - Convert 10.0m3 to units of cm3 using the...Ch. 1 - The average blood volume in the human body in...Ch. 1 - The average density of human blood is 1060kg/m3....Ch. 1 - What is the name for the change in physical state...Ch. 1 - A car traveling at 29.1 m/s drives for 2.5 hours...Ch. 1 - Prob. 146QPCh. 1 - Which of the following is an example of a...Ch. 1 - Which of the following is a pure substance that is...Ch. 1 - Which of the following statements regarding...Ch. 1 - A rectangular block of an unknown metal with a...Ch. 1 - Which of the following statement is correct? A....Ch. 1 - Which of the following statements is correct? A....Ch. 1 - Which of the following is an example of a...Ch. 1 - The number 0.00063780 correctly expressed in...Ch. 1 - Which of the following mathematical operation...Ch. 1 - Which of the following has the largest mass?...Ch. 1 - A bicyclist is traveling at 6.7 meters per second....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Given the standard enthalpies of formation for the following substances, determine the reaction enthalpy for the following reaction. 3A(g) + 1B (g) 4C (g) + 7D (g) Substance AH in kJ/mol A (g) - 25.07 B (g) - 36.51 C (g) - 90.09 D (g) + 56.11 AHran =?kJarrow_forwardWhat is the change in internal energy (ΔU) when a system is heated with 42.0 J of energy while it does 110.0 J of work?arrow_forwardCan you help me solve this problem and explain what the answers are?arrow_forward
- For which reaction below does the enthalpy change under standard conditions correspond to a standard enthalpy of formation? (Choose all that applies) SO2(g) + 1/2 O2(g) → SO3(g) 2H2(g) + C(s) → CH4(g) Mg(s) + 1/2 O2(g) → MgO(s) CO(g) + H2O(g) → CO2(g) + H2(g) CO2(g) + H2(g) → CO(g) + H2O(g) 1/2 H2(g) + 1/2 N2(g) + 3/2 O2(g) → HNO3(g) CO2(g) + C(s) 2CO(g) N2(g) + 202(g) → 2NO2(g)arrow_forwardChoose all the molecules with zero standard-enthalpy-of-formation (AH% = 0) Fe(s) FeCl2(s) N2(g) H2O(l) 02(g) C(graphite) K(s) H2O(g)arrow_forward8.5 g of potassium hydroxide (molar mass = 56.1 g/mol) dissolves in 125 g of water and the temperature of the solution increases by 15.58°C. Calculate the AH soln for potassium hydroxide. Assume the specific heat capacity of the solution is 4.2 J.g¨¹.ºC-1. KOH(s) → →K+ K(aq) + OH AH solution = ?kJ/mol (aq)arrow_forward
- What will be the final temperature of a 8.79 g piece of iron (CP = 25.09 J/(mol · oC)) initially at 25.0oC, if it is supplied with 302.8 J from a stove?arrow_forwardIdentify the set of stoichiometric coefficients that balances the reaction equation for the combustion of the hydrocarbon below: _ C19 H4002 → CO2 + H2Oarrow_forwardThe cooling system in an automobile holds 11.3 L of ethylene glycol antifreeze. How much energy is absorbed when the temperature of the ethylene glycol goes from 20oC to 100oC? The density and specific heat capacity of ethylene glycol are 1.11 g/mL and 2.42 J/(g ⋅ oC), respectively.arrow_forward
- Which statement about the following chemical reaction is not correct? 2NH3+202 →→→ N2O + 3H₂O ○ It requires 2 mol of ammonia to produce 3 mol of water. It requires 2 mol of dioxygen to produce 1 mol of N2O. ○ Nine moles of water are produced when four moles of ammonia are consumed. Two moles of N2O would be produced when four moles of dioxygen are consumed. Two moles of ammonia react with two moles of dioxygen.arrow_forwardIf 169.7 g of NaOH (40.0 g/mol) were used to prepare 3411.0 mL of solution, what would the concentration be? Group of answer choicesarrow_forwardThe mass of 3.6 mol of some element is 576 g. What is the element?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning  Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Types of Matter: Elements, Compounds and Mixtures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=dggHWvFJ8Xs;License: Standard YouTube License, CC-BY