ta... M Question 6 of 7-16 - Achieve - Pre-Lab Integration Hub i Assignment Score: 78% Resources < Question 6 of 7 > Macmillan Learning is propionic acid a salt - Google Search Give Up? Feedback Resume Attempt 4 You are asked to prepare 500. mL 0.300 M acetate buffer at pH 5.10 using only pure acetic acid (MW = 60.05 g/mol, pKa =4.76), 3.00 M NaOH, and water. How many grams s of acetic acid will be needed to prepare the 500. mL buffer? Note that the given concentration of acetate refers to the concentration of all acetate species in solution. 8.23 mass: Incorrect go
ta... M Question 6 of 7-16 - Achieve - Pre-Lab Integration Hub i Assignment Score: 78% Resources < Question 6 of 7 > Macmillan Learning is propionic acid a salt - Google Search Give Up? Feedback Resume Attempt 4 You are asked to prepare 500. mL 0.300 M acetate buffer at pH 5.10 using only pure acetic acid (MW = 60.05 g/mol, pKa =4.76), 3.00 M NaOH, and water. How many grams s of acetic acid will be needed to prepare the 500. mL buffer? Note that the given concentration of acetate refers to the concentration of all acetate species in solution. 8.23 mass: Incorrect go
Oh no! Our experts couldn't answer your question.
Don't worry! We won't leave you hanging. Plus, we're giving you back one question for the inconvenience.
Submit your question and receive a step-by-step explanation from our experts in as fast as 30 minutes.
You have no more questions left.
Message from our expert:
It looks like you may have submitted a graded question that, per our Honor Code, experts cannot answer. We've credited a question to your account.
Your Question:
Transcribed Image Text:ta...
M Question 6 of 7-16 - Achieve - Pre-Lab
Integration Hub
i Assignment Score:
78%
Resources
<
Question 6 of 7
>
Macmillan Learning
is propionic acid a salt - Google Search
Give Up?
Feedback
Resume
Attempt 4
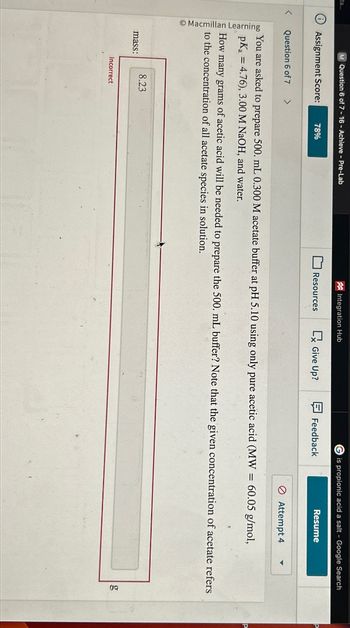
You are asked to prepare 500. mL 0.300 M acetate buffer at pH 5.10 using only pure acetic acid (MW = 60.05 g/mol,
pKa =4.76), 3.00 M NaOH, and water.
How many grams
s of acetic acid will be needed to prepare the 500. mL buffer? Note that the given concentration of acetate refers
to the concentration of all acetate species in solution.
8.23
mass:
Incorrect
go
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
