X₁0 mol -TA dm³-min 1.0 (dm³-min) mol -TA FAO (dm³-min) -TA mol 0.2 1.67 0.4 5.0 0.45 5.0 0.5 5.0 0.6 5.0 Feed Flow Rate = 300- mol sec Pure A Reaction ASTILBene → BTROSPHOPHENE + CMETHANE 0.8 Adiabatic & Exothermic 1.25 0.9 0.91
X₁0 mol -TA dm³-min 1.0 (dm³-min) mol -TA FAO (dm³-min) -TA mol 0.2 1.67 0.4 5.0 0.45 5.0 0.5 5.0 0.6 5.0 Feed Flow Rate = 300- mol sec Pure A Reaction ASTILBene → BTROSPHOPHENE + CMETHANE 0.8 Adiabatic & Exothermic 1.25 0.9 0.91
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
plz helpD

Transcribed Image Text:What final conversion can be achieved if a 72-dm^3 PFR is followed in series by a 24-dm^3 CSTR?

Transcribed Image Text:X₁0
mol
-TA dm³.min. 1.0
dm³-min
-TA
mol
FAO (dm³-min
-TA
mol
0.2
1.67
0.4
5.0
0.45
5.0
0.5
Feed Flow Rate
5.0
0.6
= 300-
5.0
Reaction ASTILBENE → BTROSPHOPHENE + CMETHANE
mol
sec PureA
0.8
Adiabatic & Exothermic
1.25
0.9
0.91
Expert Solution
Step 1: Conversion in PFR followed by CSTR
Given, feed flow rate of A
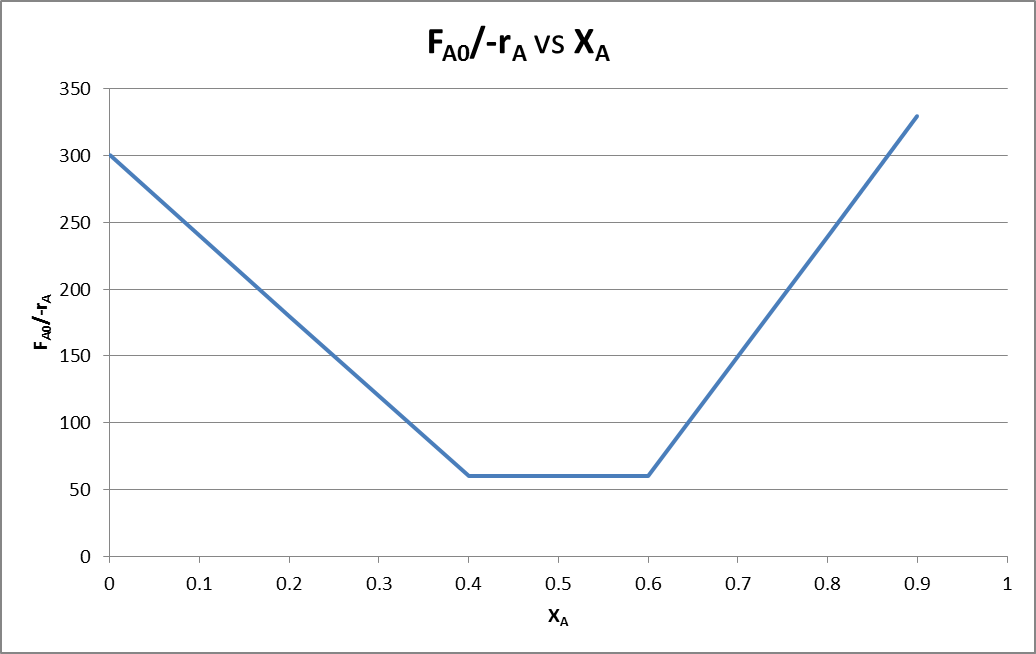
Now we tabulate data of conversion of A (XA) versus using the table provided.
| XA | |
| 0 | 300 |
| 0.2 | 179.6407 |
| 0.4 | 60 |
| 0.45 | 60 |
| 0.5 | 60 |
| 0.6 | 60 |
| 0.8 | 240 |
| 0.9 | 329.6703 |
Now we will plot curve between XA and FA0/-rA using Microsoft Excel.
Step by step
Solved in 4 steps with 14 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The