Which one (if any) of the following half-cells will be the anode when paired with a Zn2+(0.1 M)/Zn half-cell in a galvanic cell? Al3+(0.1 M)/Al reduction potential = −1.68 V None of these half-cells will be the anode. Cu2+(0.1 M)/Cu reduction potential = +0.31 V Fe3+(0.1 M)/Fe reduction potential = −0.04 V
Which one (if any) of the following half-cells will be the anode when paired with a Zn2+(0.1 M)/Zn half-cell in a galvanic cell?
- Al3+(0.1 M)/Al
- reduction potential = −1.68 V
- None of these half-cells will be the anode.
- Cu2+(0.1 M)/Cu
- reduction potential = +0.31 V
- Fe3+(0.1 M)/Fe
- reduction potential = −0.04 V
Galvanic cell : It is a electrochemical cell where chemical energy gives electrical energy.
Standard reduction potential : - when a particular ion gains some electron then this process is called reduction & the potential generated is called standard reduction potential.
Lets consider the half cell reaction of Zn2+/Zn couple :
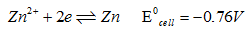
Concept :- Between the two half cell , where the reduction potential value is lower , that will acts as the anode & at anode oxidation reaction takes place.
case -1: Al3+(0.1 M)/Al, reduction potential = −1.68 V & Zn2+(0.1M)/Zn reduction potential =-0.76 V.
Al3+(0.1 M)/Al reduction potential is lower than Zn2+(0.1M)/Zn
So, Al3+(0.1 M)/Al will definitely acts as an anode here. (answer)
Step by step
Solved in 2 steps with 1 images









