O KINETICS AND EQUILIBRIUM = Valentina V Solving problems that mix equilibrium ideas with gas laws Sulfuric acid is essential to dozens of important industries from steelmaking to plastics and pharmaceuticals. More sulfuric acid is made than any other industrial chemical, and world production exceeds 2.0 × 10¹¹ kg per year. The first step in the synthesis of sulfuric acid is usually burning solid sulfur to make sulfur dioxide gas. Suppose an engineer studying this reaction introduces 1.5 kg of solid sulfur and 10.0 atm of oxygen gas at 400. °C into an evacuated 85.0 L tank. The engineer believes K₂=0.087 for the reaction at this temperature. Calculate the mass of solid sulfur she expects to be consumed when the reaction reaches equilibrium. Round your answer to 2 significant digits. alo Note for advanced students: the engineer may be mistaken in her belief about the value of K, and the consumption of sulfur you calculate may not be what she actually observes. 9 kg Ś ? 0.2 X
O KINETICS AND EQUILIBRIUM = Valentina V Solving problems that mix equilibrium ideas with gas laws Sulfuric acid is essential to dozens of important industries from steelmaking to plastics and pharmaceuticals. More sulfuric acid is made than any other industrial chemical, and world production exceeds 2.0 × 10¹¹ kg per year. The first step in the synthesis of sulfuric acid is usually burning solid sulfur to make sulfur dioxide gas. Suppose an engineer studying this reaction introduces 1.5 kg of solid sulfur and 10.0 atm of oxygen gas at 400. °C into an evacuated 85.0 L tank. The engineer believes K₂=0.087 for the reaction at this temperature. Calculate the mass of solid sulfur she expects to be consumed when the reaction reaches equilibrium. Round your answer to 2 significant digits. alo Note for advanced students: the engineer may be mistaken in her belief about the value of K, and the consumption of sulfur you calculate may not be what she actually observes. 9 kg Ś ? 0.2 X
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
100%

Transcribed Image Text:O KINETICS AND EQUILIBRIUM
Valentina V
=
0/5
Solving problems that mix equilibrium ideas with gas laws
Sulfuric acid is essential to dozens of important industries from steelmaking to plastics and pharmaceuticals. More sulfuric acid is made than any other industrial
chemical, and world production exceeds 2.0 × 10¹¹ kg per year.
The first step in the synthesis of sulfuric acid is usually burning solid sulfur to make sulfur dioxide gas. Suppose an engineer studying this reaction introduces
1.5 kg of solid sulfur and 10.0 atm of oxygen gas at 400. °C into an evacuated 85.0 L tank. The engineer believes Kp = 0.087 for the reaction at this
temperature.
Calculate the mass of solid sulfur she expects to be consumed when the reaction reaches equilibrium. Round your answer to 2 significant digits.
alo
Note for advanced students: the engineer may be mistaken in her belief about the value of K, and the consumption of sulfur you calculate may not be what
she actually observes.
Ar
kg
x10
?
Explanation
© 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
Check
X
Ś
A
KI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
what's this one?
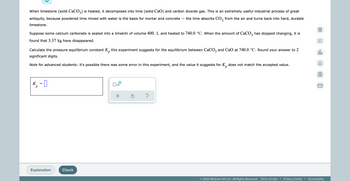
Transcribed Image Text:When limestone (solid CaCO3) is heated, it decomposes into lime (solid CaO) and carbon dioxide gas. This is an extremely useful industrial process of great
antiquity, because powdered lime mixed with water is the basis for mortar and concrete the lime absorbs CO₂ from the air and turns back into hard, durable
2
limestone.
Suppose some calcium carbonate is sealed into a limekiln of volume 400. L and heated to 740.0 °C. When the amount of CaCO3 has stopped changing, it is
found that 3.37 kg have disappeared.
P
00.
Calculate the pressure equilibrium constant K this experiment suggests for the equilibrium between CaCO3 and CaO at 740.0 °C. Round your answer to 2
significant digits.
Ar
Note for advanced students: it's possible there was some error in this experiment, and the value it suggests for K does not match the accepted value.
р
K₁ = 0
x10
р
x Ś
?
Explanation
Check
0
81
K
© 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY