What Volume (PFR) is required to obtain 96% for the single reactor process. this is what i have done so far not sure if i am in right direction regression rate law was given as a known
What Volume (PFR) is required to obtain 96% for the single reactor process. this is what i have done so far not sure if i am in right direction regression rate law was given as a known
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
100%
What Volume (PFR) is required to obtain 96% for the single reactor process.
this is what i have done so far not sure if i am in right direction
regression rate law was given as a known

Transcribed Image Text:dFA
dV
CAO = 0.144 gmol * L-¹
YAO = 0.5
УАО
Vo = 6L * S-1
V = ?
XA =?
FAO
= TA
(dXA)
= fav
1
-dXA
-rAexit
FAO
FAO
(-A) exitdXA
XA
= S²²
= -TA
dV → FAO
=
=f₁dv.
dV
.XA
1
-TAexit
0
FA0o = √²
dXA
XA
TA =
- fº av →
=
T = 422.2K
P = 10 atm
0.00596(1-XA) 0.94671
FAO = : CAovo
1
0.00596(1 – Xa)0.94671
dXA = V

Transcribed Image Text:FAO =
.XA
0
167.785(1-X)-0.94671dX
dXA = V
Expert Solution
Step 1: Design equation for the Ideal PFR
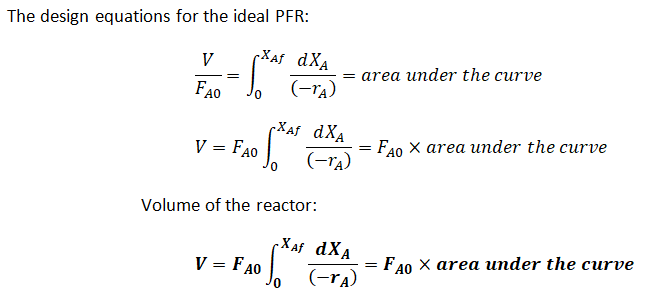
Step by step
Solved in 4 steps with 6 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The