
•Here firstly we understand about "Conformer".
•Conformer:- Conformers are stereoisomers which are inter convertible just by rotation of atoms on a axis.
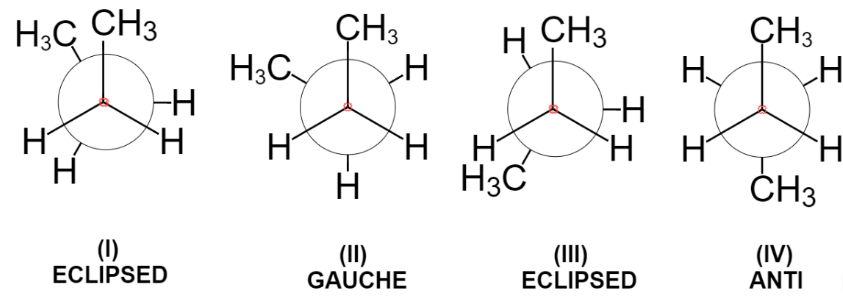
In eclipsed conformation(I) the two methyl groups are one behind another. This is maximum energy conformation and thus most unstable due to steric repulsion between two bulky methyl groups.
Now, turning the front carbon by 60 degrees clockwise, we get the gauche conformation in which two methyl groups are somewhat far apart still the repulsion between two exists.
Further rotation of 60 degrees results again in the formation of eclipsed conformation in which methyl groups are behind the hydrogen atoms. Due to steric repulsion between methyl and hydrogen, this conformation is less stable than gauche but more stable than eclipsed conformation (I).
Again, rotation of 60 degrees gives the anti-conformation where the two bulky groups are situated opposite to each other and thus it is the most stable conformation due to minimum steric repulsion.
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images









