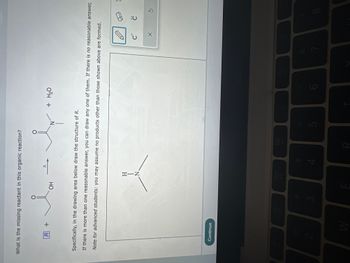
Check the box under each maece that is an Isomer of this moewe w ~ Dor none of the above I what is the missing reactant in this reaction? مسائل OH N + H₂O draw structux of /R if no answer click no ansua
Reactive Intermediates
In chemistry, reactive intermediates are termed as short-lived, highly reactive atoms with high energy. They rapidly transform into stable particles during a chemical reaction. In specific cases, by means of matrix isolation and at low-temperature reactive intermediates can be isolated.
Hydride Shift
A hydride shift is a rearrangement of a hydrogen atom in a carbocation that occurs to make the molecule more stable. In organic chemistry, rearrangement of the carbocation is very easily seen. This rearrangement can be because of the movement of a carbocation to attain stability in the compound. Such structural reorganization movement is called a shift within molecules. After the shifting of carbocation over the different carbon then they form structural isomers of the previous existing molecule.
Vinylic Carbocation
A carbocation where the positive charge is on the alkene carbon is known as the vinyl carbocation or vinyl cation. The empirical formula for vinyl cation is C2H3+. In the vinyl carbocation, the positive charge is on the carbon atom with the double bond therefore it is sp hybridized. It is known to be a part of various reactions, for example, electrophilic addition of alkynes and solvolysis as well. It plays the role of a reactive intermediate in these reactions.
Cycloheptatrienyl Cation
It is an aromatic carbocation having a general formula, [C7 H7]+. It is also known as the aromatic tropylium ion. Its name is derived from the molecule tropine, which is a seven membered carbon atom ring. Cycloheptatriene or tropylidene was first synthesized from tropine.
Stability of Vinyl Carbocation
Carbocations are positively charged carbon atoms. It is also known as a carbonium ion.
![O
#8
Check the box under each maecul that is
an Isomer of this moewe
Dor none of the above.
#9) Jun
на
W
R] +
+
what is the missing reactant in this reaction
он
□
AX
2-
+ H₂O
draw structure of TRI if no answer click no answer
C](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Ff2ad28d4-57e0-4bac-8987-e1e814b64f4e%2Fe9be1b61-e898-4b29-962a-77d9fe096ee4%2Fp9lcxow_processed.jpeg&w=3840&q=75)
Step by step
Solved in 2 steps with 1 images

Question on the second question , I attached a picture to see if that's the way to draw it or not !
is it right the way I drew it ?
Thanks