Formal Charges
Formal charges have an important role in organic chemistry since this concept helps us to know whether an atom in a molecule is neutral/bears a positive or negative charge. Even if some molecules are neutral, the atoms within that molecule need not be neutral atoms.
Polarity Of Water
In simple chemical terms, polarity refers to the separation of charges in a chemical species leading into formation of two polar ends which are positively charged end and negatively charged end. Polarity in any molecule occurs due to the differences in the electronegativities of the bonded atoms. Water, as we all know has two hydrogen atoms bonded to an oxygen atom. As oxygen is more electronegative than hydrogen thus, there exists polarity in the bonds which is why water is known as a polar solvent.
Valence Bond Theory Vbt
Valence bond theory (VBT) in simple terms explains how individual atomic orbitals with an unpaired electron each, come close to each other and overlap to form a molecular orbital giving a covalent bond. It gives a quantum mechanical approach to the formation of covalent bonds with the help of wavefunctions using attractive and repulsive energies when two atoms are brought from infinity to their internuclear distance.
What is a Brief summary of the six steps used to analyze reactions with pi bonds ?
It is important to train our eye to recognize structural features that have stabilizing effects. Alternating single and double bonds create a conjugated pi bond system across multiple atoms that lowers the energy and stabilizes the molecule or ion. When we look at carbon-carbon double bonds (C=C), we need to look and see if they are isolated or conjugated.
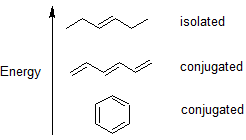
To understand the source of this stabilization we will use molecular orbital (MO) theory. Valence bond theory does a remarkably good job at explaining the bonding geometry of many of the functional groups in organic compounds, however, it fails to adequately account for the stability contained in alternating double and single bonds. In order to understand these properties, we will use the ideas of MO theory.
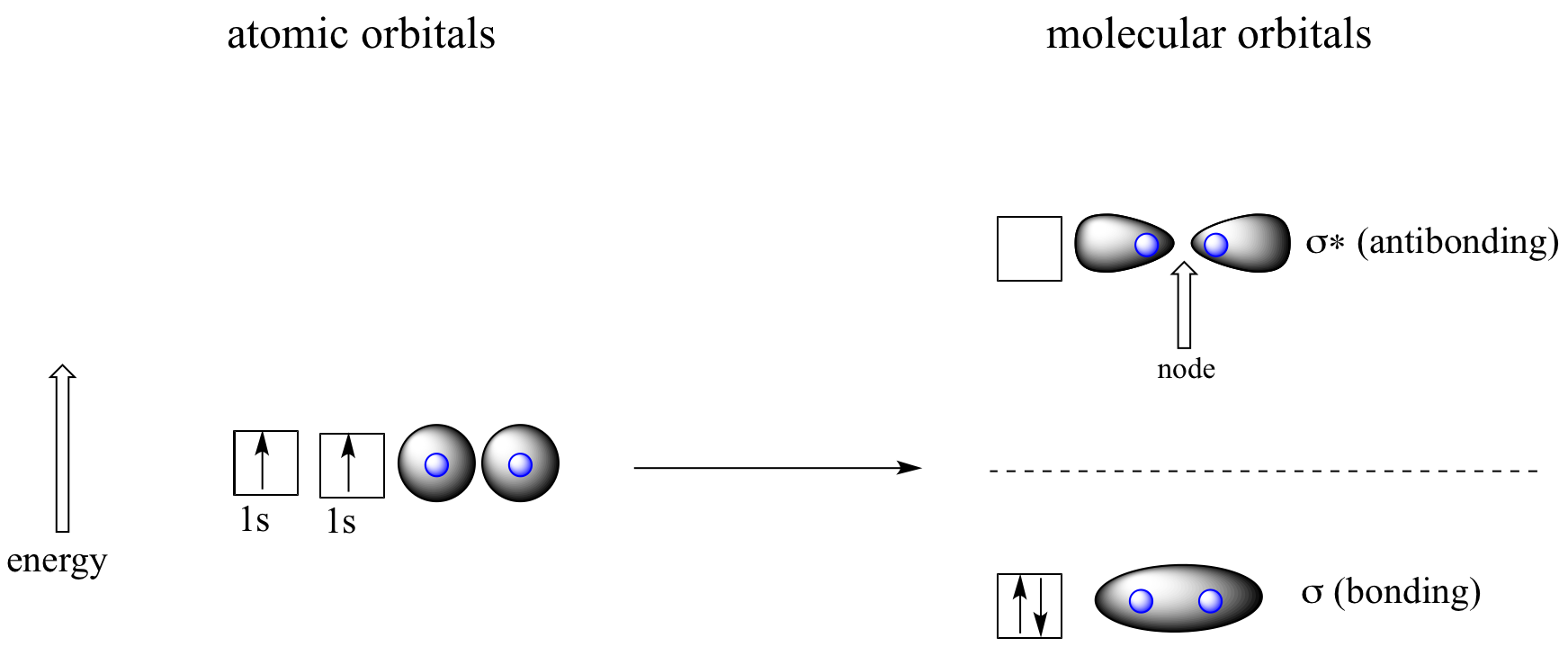
Let’s go back and consider again the simplest possible covalent bond: the one in molecular hydrogen (H2). When we described the hydrogen molecule using valence bond theory, we said that the two 1s orbitals from each atom overlap, allowing the two electrons to be shared and thus forming a covalent bond. In molecular orbital theory, we make a further statement: we say that the two atomic 1s orbitals mathematically combine to form two new orbitals. Recall that an atomic orbital (such as the 1s orbital of a hydrogen atom) describes a region of space around a single atom inside which electrons are likely to be found. A molecular orbital describes a region of space around two or more atoms inside which electrons are likely to be found.
Mathematical principles tell us that when orbitals combine, the number of orbitals before the combination takes place must equal the number of new orbitals that result from the combination – orbitals don’t just disappear! We saw this previously when we discussed hybrid orbitals: one s and three p orbitals make four sp3 hybrids. When two atomic 1s orbitals combine in the formation of H2, the result is two sigma (σ) orbitals.
Step by step
Solved in 2 steps with 9 images









