A reaction B is to be carried out isothermally in a continuous flow reactor. The entering volumetric flow rate = 10 dm^3/hour. The feed molar flow rate FAO = 6 mol/hour. Calculate both the CSTR reactor volume and the PFR reactor volume that will achieve 96% consumption of A (i.e. CA = 0.04*CAO) and assuming the reaction rate -rA is: -rA = k (b)-ra = k*CA (c)-rA = k*CA² A with k = 0.040 mol/(hr*dm^3) with k = 0.00012 sec¹ with k = 320 dm^3/(mol*hr)
A reaction B is to be carried out isothermally in a continuous flow reactor. The entering volumetric flow rate = 10 dm^3/hour. The feed molar flow rate FAO = 6 mol/hour. Calculate both the CSTR reactor volume and the PFR reactor volume that will achieve 96% consumption of A (i.e. CA = 0.04*CAO) and assuming the reaction rate -rA is: -rA = k (b)-ra = k*CA (c)-rA = k*CA² A with k = 0.040 mol/(hr*dm^3) with k = 0.00012 sec¹ with k = 320 dm^3/(mol*hr)
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question

Transcribed Image Text:A reaction
A
B
is to be carried out isothermally in a continuous flow reactor. The entering volumetric flow rate v = 10
dm^3/hour. The feed molar flow rate FAO = 6 mol/hour. Calculate both the CSTR reactor volume and the
PFR reactor volume that will achieve 96% consumption of A (i.e. CA = 0.04*CAO) and assuming the
reaction rate -rA is:
(a) -rA = k
(b)-rA = k*CA
(c)-ra = k*CA²
with k = 0.040 mol/(hr*dm^3)
with k = 0.00012 sec¹¹
with k = 320 dm^3/(mol*hr)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 11 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
how did you derive the equations for part B & C
i dont know how you did that
so orignally we have V=(v0Ca0-vCa)/(-rA) and i see how we rearranged that to get Ca0-Ca=k(v/v0) but i dont know how you got ln (1-x)=-kV/V0 or X/1-X=kCa0(V/vo)
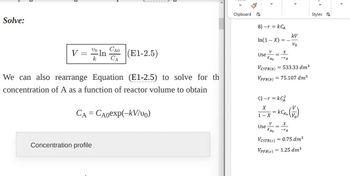
Transcribed Image Text:Solve:
vo CAO
V = ln
k CA
(E1-2.5)
We can also rearrange Equation (E1-2.5) to solve for th
concentration of A as a function of reactor volume to obtain
CA = CAoexp(-kV/vo)
Concentration profile
Clipboard
B) -r = KCA
In(1-X) = -
V
Use =
FAO
<
VCSTR(b)
VPFR(b)
kV
Vo
X
-TA
= 533.33 dm³
= 75.107 dm³
C) - r= kC²
X
1 x x = KCA. (1)
- X
Use=
X
-TA
VCSTR(C) = 0.75 dm³
VPFR(C) = 1.25 dm³
5 >
Styles
<
Solution
Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The