(w) -r( ) =T Ꭷ-p ᎧᎢ2 ᎧᏙ T V
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
![**Transcription:**
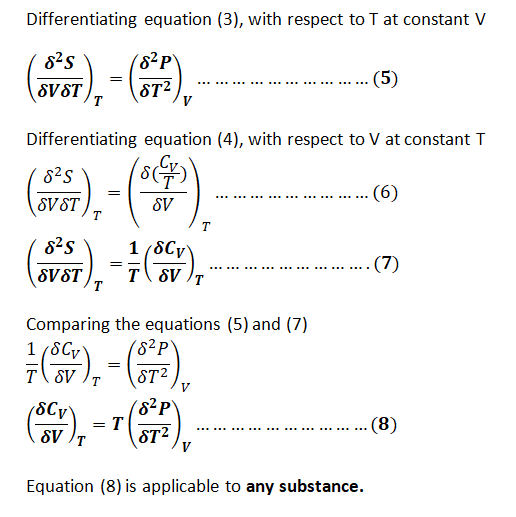
Show that
\[
\left( \frac{\partial C_v}{\partial V} \right)_T = T \left( \frac{\partial^2 p}{\partial T^2} \right)_V,
\]
and evaluate this quantity for a van der Waals gas.
---
**Explanation:**
This equation explores the relationship between the partial derivative of the heat capacity at constant volume \((C_v)\) with respect to volume \((V)\) at constant temperature \((T)\), and another partial derivative involving the pressure \((p)\) with respect to temperature \((T)\) at constant volume. This relationship is an important concept in thermodynamics, particularly when analyzing real gases like the van der Waals gas. The equation can be used to evaluate how changes in volume affect the heat capacity at constant volume for a van der Waals gas by relating it to temperature and pressure variations.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F9d6b1f03-b4bd-4f60-ae23-d8256607755b%2F59f6769a-2a5c-4f3b-b839-c740c0a3e972%2Fipej5zy_processed.png&w=3840&q=75)
Transcribed Image Text:**Transcription:**
Show that
\[
\left( \frac{\partial C_v}{\partial V} \right)_T = T \left( \frac{\partial^2 p}{\partial T^2} \right)_V,
\]
and evaluate this quantity for a van der Waals gas.
---
**Explanation:**
This equation explores the relationship between the partial derivative of the heat capacity at constant volume \((C_v)\) with respect to volume \((V)\) at constant temperature \((T)\), and another partial derivative involving the pressure \((p)\) with respect to temperature \((T)\) at constant volume. This relationship is an important concept in thermodynamics, particularly when analyzing real gases like the van der Waals gas. The equation can be used to evaluate how changes in volume affect the heat capacity at constant volume for a van der Waals gas by relating it to temperature and pressure variations.
Expert Solution
Step 1: Effect of volume on Cv at constant T for any substance
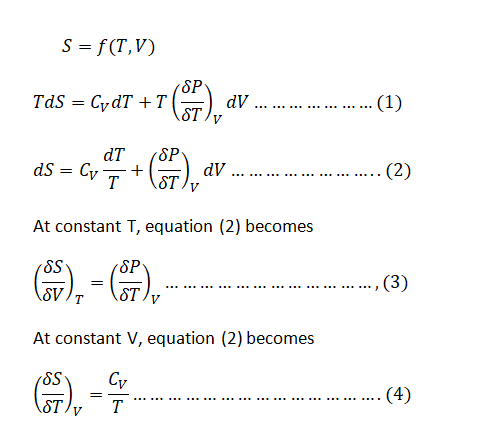
Step by step
Solved in 4 steps with 7 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The