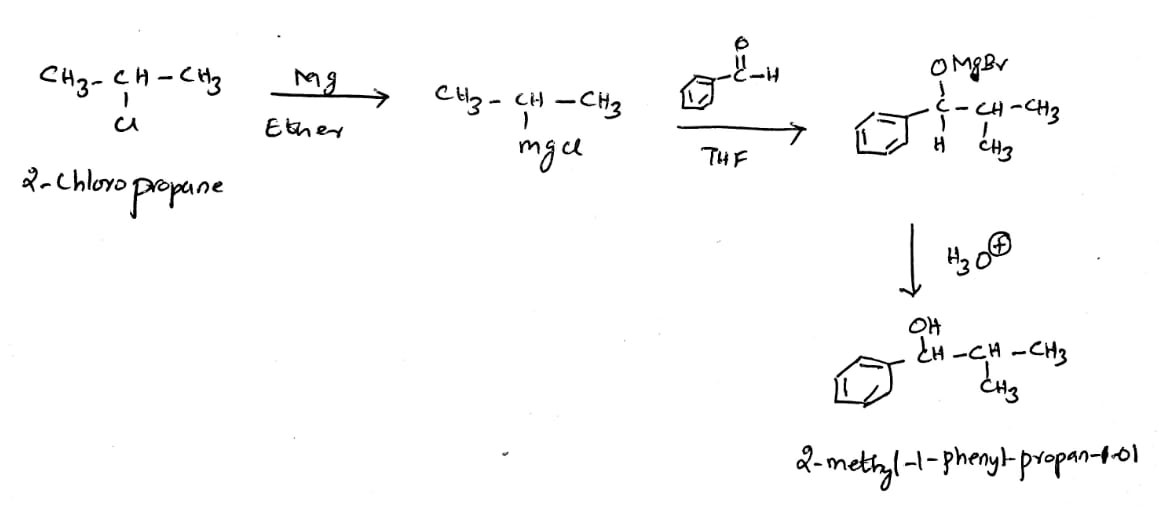
Use the Grignard reagent in 2-chioropropane , write a chemical equation to show the product produced when benzaldehyde is added, a usual work-up is done.
(c) Use the Grignard reagent in 2-chioropropane , write a chemical equation to show the product produced when benzaldehyde is added, a usual work-up is done.
The Grignard reagent is denoted as R-MgX, Where R is an alkyl or aryl group and X is a halogen such as chlorine, bromine, iodine. The reagent has a high nucleophilicity and adds across the carbonyl compounds to furnish the nucleophilic addition products such as alcohol. In primary alcohol, the -OH bonded carbon has a directly bonded carbon. In secondary and tertiary alcohols, the -OH bonded carbon has two and three directly bonded carbon atoms.
The reaction of 2-chloropropane with magnesium in dry ether furnishes the corresponding Grignard reagent with -MgCl unit. The alkyl group is nucleophilic in nature and has a high electron-rich carbon center. When the alkyl magnesium chloride is treated with benzaldehyde, the nucleophilic addition of the Grignard reagent to the benzaldehyde produces the intermediate magnesium salt. Hydrolysis of the magnesium salt furnishes the corresponding secondary alcohol.
Step by step
Solved in 3 steps with 1 images









