Use Table to write a balanced formation equation at standard conditions for each of the following com-pounds: (a) HI; (b) SiF₄; (c) O₃; (d) Ca₃(PO₄)₂.
Use Table to write a balanced formation equation at standard conditions for each of the following com-pounds: (a) HI; (b) SiF₄; (c) O₃; (d) Ca₃(PO₄)₂.
Since you have posted a question with multiple sub-parts we will solve the first three for you. To get the remaining sub-parts solved, please repost the complete question and mention the sub-parts to be solved.
We have to write a balanced equation for the formation of the HI, SiF4 and O3 under standard temperature and pressure (STP) conditions.
Answer for the first sub-part (a):
Formation of HI: There are many ways to prepare hydrogen iodide. The most easiest way is:
When hydrogen and iodine are mixed in gaseous states at standard temperature and pressure it results in the formation of hydrogen iodide as shown below:
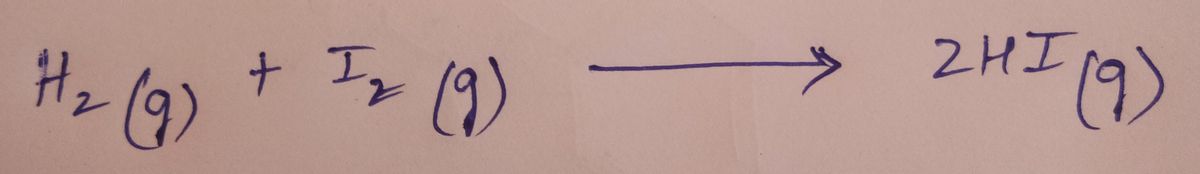
Step by step
Solved in 4 steps with 3 images









