Use LeChatelier’s principle to describe the effect on the equilibrium mixture (shift left, shift right, no shift) when the following reaction conditions are changed. N2 (g) + 3 H2 (g) ⇌ 2 NH3(g) H = - 22 kcal/mol a) Removing some NH3 (b) Decreasing the pressure (c) Decreasing the temperature (d) Adding some N2 (e) Add a catalyst
Use LeChatelier’s principle to describe the effect on the equilibrium mixture (shift left, shift right, no shift) when the following reaction conditions are changed.
N2 (g) + 3 H2 (g) ⇌ 2 NH3(g) H = - 22 kcal/mol
a) Removing some NH3
(b) Decreasing the pressure
(c) Decreasing the temperature
(d) Adding some N2
(e) Add a catalyst
Hey, since there are multiple sub-part questions posted, we will answer first three questions. If you want any specific question to be answered then please submit that question only or specify the question number in your message
According to Le Chateliers principle, when an equilibrium system is disturbed by changing conditions, the equilibrium position will shift in a direction that reduces or cancels the change.
The reaction given is:
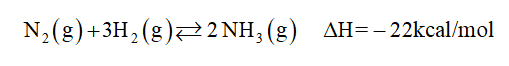
(a)
Given that some ammonia is being removed.
When ammonia is removed, system will try to increase the concentration of ammonia. Therefore, equilibrium will shift towards forward reaction (right ).
Step by step
Solved in 5 steps with 1 images









