Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
100%
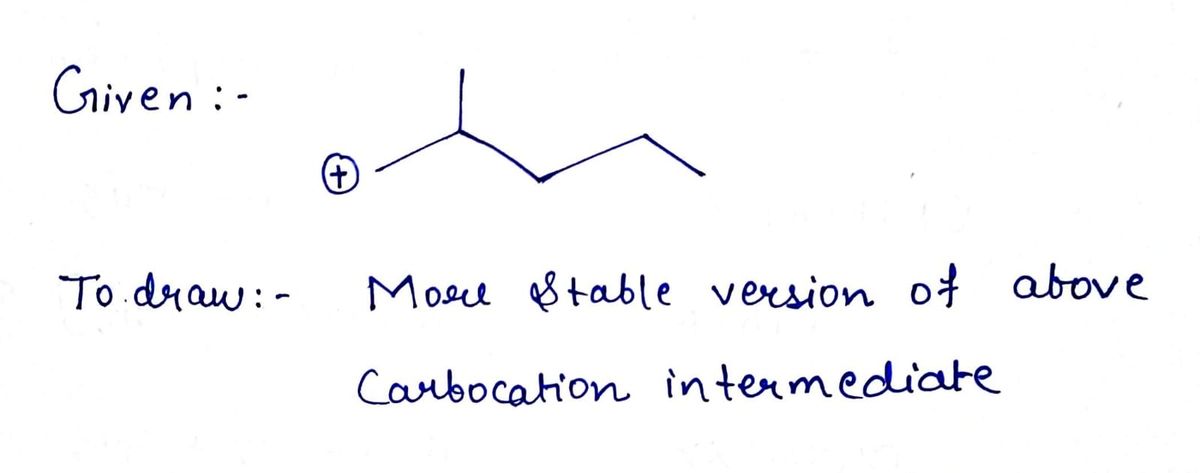
Use curved arrows to draw a more stable version of the following

Transcribed Image Text:The image depicts a chemical structure, specifically a molecule featuring a carbon backbone. It is represented in a zigzag line structure which is commonly used to denote carbon atoms and their chemical bonds in organic chemistry.
### Description of the Structure:
1. **Zigzag Line**:
- The zigzag pattern indicates a chain of carbon atoms. Each vertex, end, or sharp angle in the line typically represents a carbon atom.
2. **Positive Charge**:
- A circled "+" sign is present on the leftmost line, indicating that part of the molecule is positively charged. This suggests the presence of a carbocation, meaning one of the carbon atoms has lost an electron and carries a positive charge.
3. **Branching**:
- The vertical line extending upwards from the carbon chain suggests branching, which means an additional carbon or functional group is connected to the main carbon chain.
This kind of diagram is used in organic chemistry to simplify the representation of complex molecular structures, making it easier to understand the connectivity and arrangement of atoms within the molecule.
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY