thermodynamic equation of state:
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
p5c
![**Question 5**
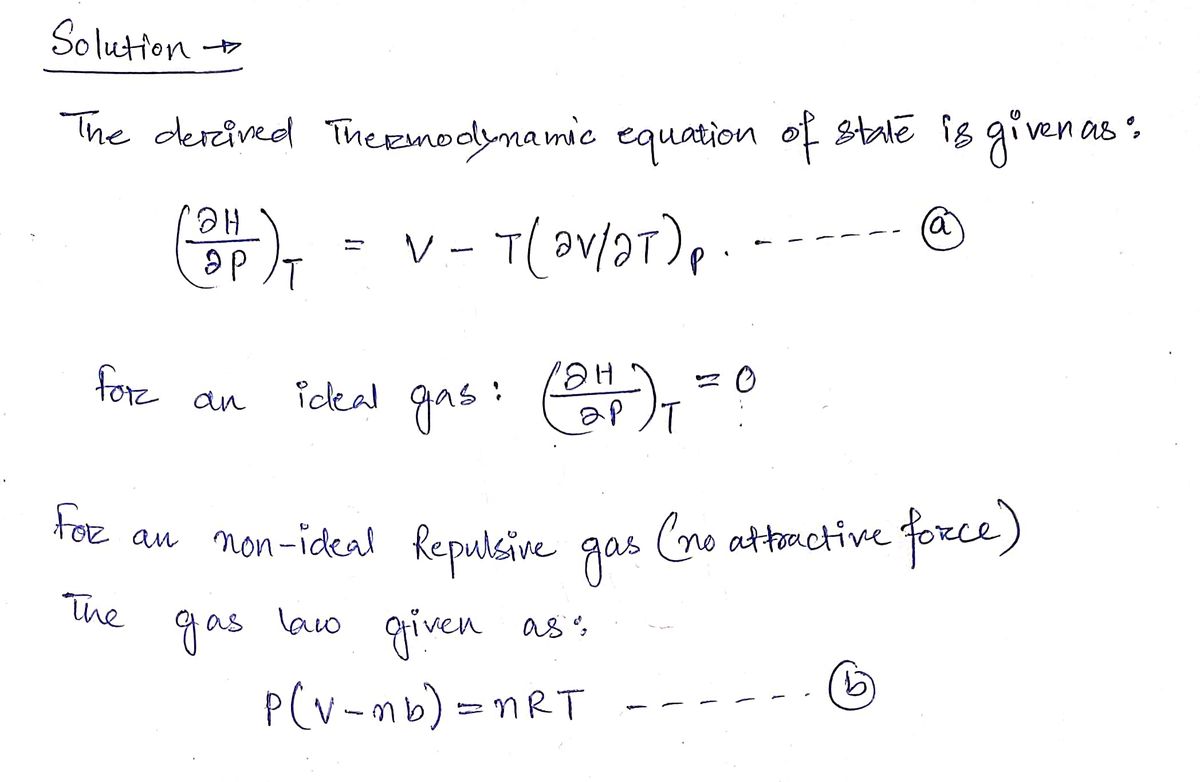
We have derived the thermodynamic equation of state:
\[
\left( \frac{\partial H}{\partial p} \right)_T = V - T \left( \frac{\partial V}{\partial T} \right)_p
\]
and we showed that for an ideal gas this becomes zero:
\[
\left( \frac{\partial H}{\partial p} \right)_T = 0
\]
Please go over this part before working on the following question.
Suppose a gas is not ideal but has repulsive interactions (no attractive force), so the gas law is given by:
\[
p(V-nb) = nRT
\]
Find
\[
\left( \frac{\partial H}{\partial p} \right)_T = ?
\]
Please enter an equation in terms of \( p, V, T, n, b \).
[Answer Box]](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Ff4dfc165-1571-419f-99fc-a4587d76c340%2Fb05949dd-c84c-4264-b944-36fc3ce80626%2Fa4bazu1_processed.jpeg&w=3840&q=75)
Transcribed Image Text:**Question 5**
We have derived the thermodynamic equation of state:
\[
\left( \frac{\partial H}{\partial p} \right)_T = V - T \left( \frac{\partial V}{\partial T} \right)_p
\]
and we showed that for an ideal gas this becomes zero:
\[
\left( \frac{\partial H}{\partial p} \right)_T = 0
\]
Please go over this part before working on the following question.
Suppose a gas is not ideal but has repulsive interactions (no attractive force), so the gas law is given by:
\[
p(V-nb) = nRT
\]
Find
\[
\left( \frac{\partial H}{\partial p} \right)_T = ?
\]
Please enter an equation in terms of \( p, V, T, n, b \).
[Answer Box]
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The