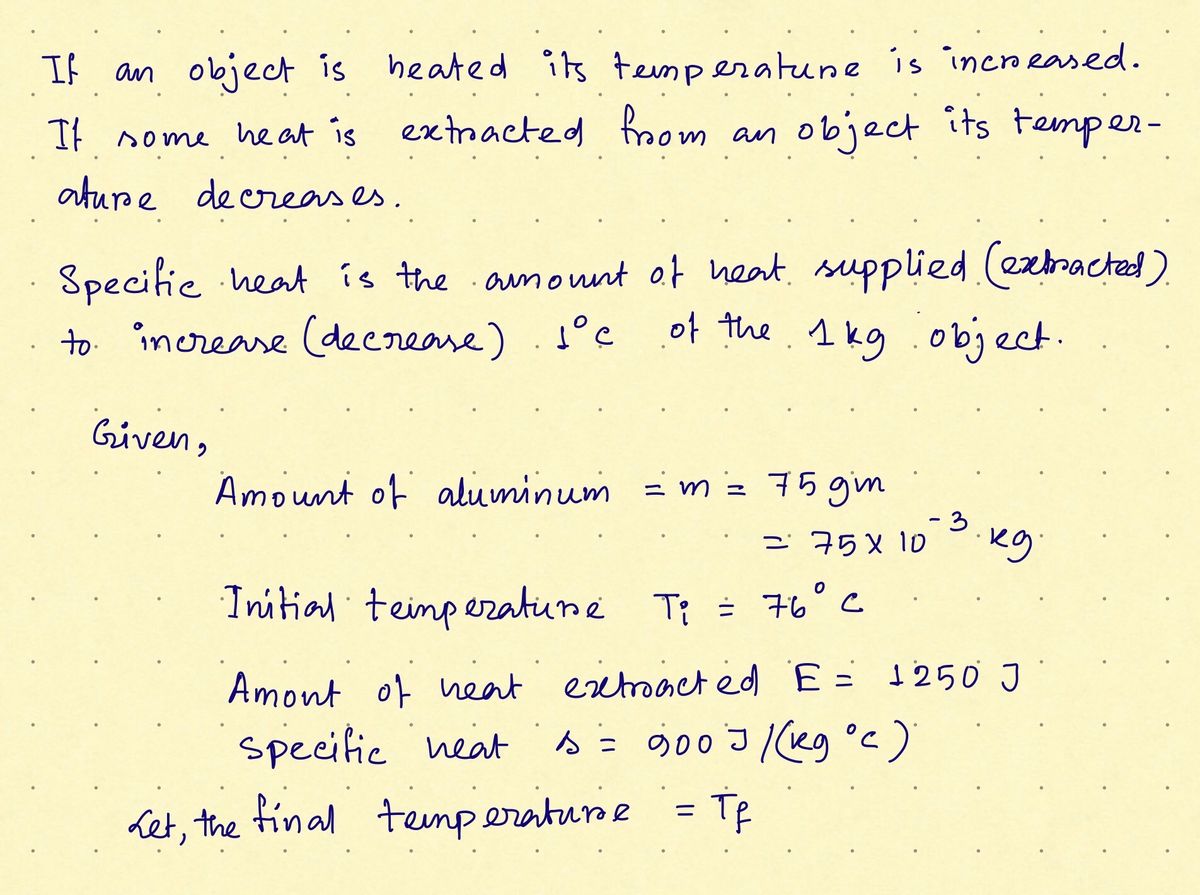
The temperature of an object is changed when heat is added to or extracted from it. Determine the final temperature (in °C) of a 75.0 g mass of aluminum initially at 76.0°C if 1,250 J of heat energy is extracted from it. The specific heat of aluminum is 900 J/(kg - °C).
The temperature of an object is changed when heat is added to or extracted from it. Determine the final temperature (in °C) of a 75.0 g mass of aluminum initially at 76.0°C if 1,250 J of heat energy is extracted from it. The specific heat of aluminum is 900 J/(kg - °C).
Related questions
Question
Very confused on the whole question. Could I get a detailed way of properly breaking down the question and getting the answer?

Transcribed Image Text:The temperature of an object is changed when heat is added to or extracted from it. Determine the final temperature (in
°C) of a 75.0 g mass of aluminum initially at 76.0°C if 1,250 J of heat energy is extracted from it. The specific heat of
aluminum is 900 J/(kg• °C).
°C
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images
