The reaction below produces a single major product. Show the mechanism for its formation, including all resonance forms for the intermediate. NO2 Br FeBr, Attach File Browse Content Collection Browse Local File
The reaction below produces a single major product. Show the mechanism for its formation, including all resonance forms for the intermediate. NO2 Br FeBr, Attach File Browse Content Collection Browse Local File
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:The reaction below produces a single major product. Show the mechanism for its formation, including all resonance forms for the intermediate.
**Diagram Description:**
A benzene ring with a nitro group (NO₂) attached to one of its carbon atoms is shown. There is an arrow indicating a reaction with Br₂ and FeBr₃. The image also includes clickable buttons labeled "Attach File," "Browse Local Files," and "Browse Content Collection."
This reaction likely involves electrophilic aromatic substitution, where the bromine will substitute a hydrogen atom on the benzene ring. Given the presence of the nitro group, which is an electron-withdrawing group, the substitution will occur at either the meta or para position relative to the nitro group, with meta being more favored. The mechanism should detail these steps and include resonance structures for the intermediate cation.
Expert Solution
Step 1
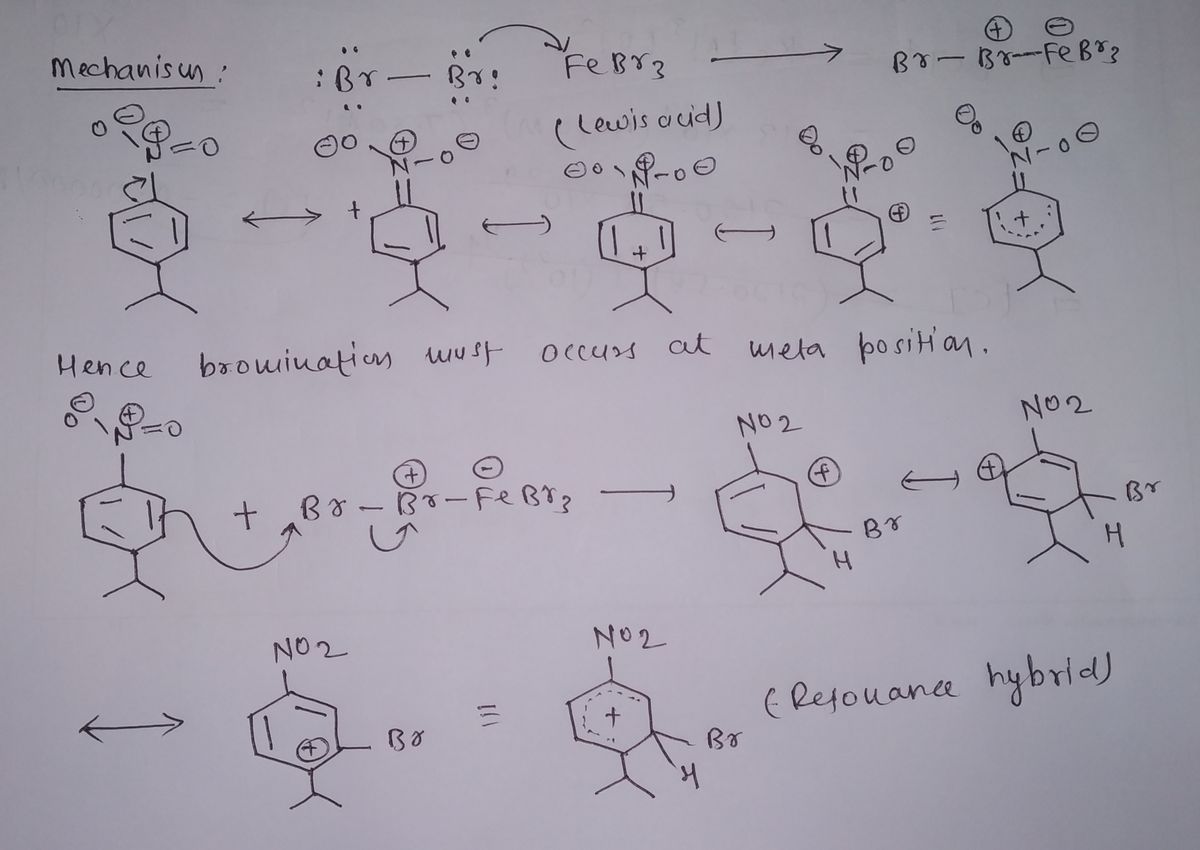
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY