The octahedral complex ion [MNCI]³¯ has more unpaired spins than the octahedral complex ion [Mn(CN),]³-. How many unpaired electrons are present in each species? Explain. In each case, express the CFSE in terms of A.
![The octahedral complex ion [MNCI]³¯ has more unpaired
spins than the octahedral complex ion [Mn(CN),]³-. How
many unpaired electrons are present in each species?
Explain. In each case, express the CFSE in terms of A.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Ff4c65388-1c42-43e5-b28c-f1d7aa437740%2F98150412-c168-49b2-b9d8-2a3d9247cbd0%2Ffyr8zil.png&w=3840&q=75)
In case of CFT, concept of strong ligand and weak ligand arises in case of pairing energy and splitting of orbital. Strong ligand have low pairing energy and high splitting energy. So, they prefer to pair electrons in t2g orbital rather to excite them. And they have very less unpaired electrons (low-spin complexes).
Weak ligand have low splitting energy and high pairing energy. So. they try to fill eg orbital after half filling of t2g orbital. And they have more numbers of unpaired electrons (high-spin complex).
[Mn(Cl)6]3- has more unpaired electrons because Cl- is a weak ligand and it does not prefer pairing of unpaired electrons. So, it forms an outer orbital complex and hybridization will be sp3d2.
Its oxidation state will be:
Mn3+:[Ar] 3d4, expressed as 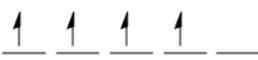 . So, it has 4 unpaired electrons, and on splitting in t2g and eg it will become as:
. So, it has 4 unpaired electrons, and on splitting in t2g and eg it will become as:
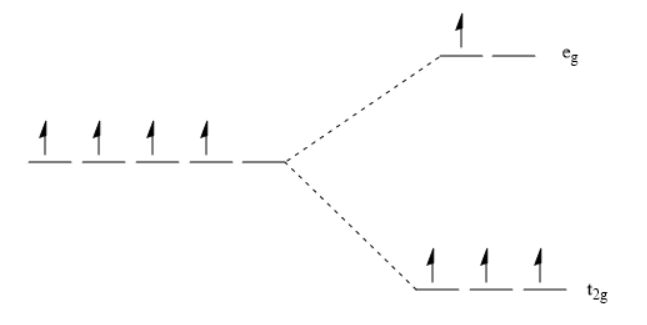
For calculating CFSE in terms of o, the formula used is:
where m is the number of electrons in t2g and n is the number of electrons in eg.
From the splitting, it is observed that m is 3 and n is 1.
Step by step
Solved in 3 steps with 4 images









