The freezing point of water, H,0, is 0.000 °C at 1 atmosphere. KAwater) = 1.86 °C/m In a laboratory experiment, students synthesized a new compound and found that when 12.93 grams of the compound were dissolved in 273.5 grams of water, the solution began to freeze at -0.488 °C. The compound was also found to be nonvolatile and a non-electrolyte. What is the molecular weight they determined for this compound? g/mol
The freezing point of water, H,0, is 0.000 °C at 1 atmosphere. KAwater) = 1.86 °C/m In a laboratory experiment, students synthesized a new compound and found that when 12.93 grams of the compound were dissolved in 273.5 grams of water, the solution began to freeze at -0.488 °C. The compound was also found to be nonvolatile and a non-electrolyte. What is the molecular weight they determined for this compound? g/mol
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
please explain
![The freezing point of water, \( \text{H}_2\text{O} \), is 0.000 °C at 1 atmosphere. \( K_f(\text{water}) = 1.86 \, \text{°C/m} \)
In a laboratory experiment, students synthesized a new compound and found that when 12.93 grams of the compound were dissolved in 273.5 grams of water, the solution began to freeze at -0.488 °C. The compound was also found to be nonvolatile and a non-electrolyte.
What is the molecular weight they determined for this compound?
\[ \_\_\_\_\_\_ \, \text{g/mol} \]](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fc4eca0ea-f525-4d8f-9259-04e892974916%2Fa38d1261-ccb0-448a-b762-8f0d59d25f3e%2Fsuc30x_processed.jpeg&w=3840&q=75)
Transcribed Image Text:The freezing point of water, \( \text{H}_2\text{O} \), is 0.000 °C at 1 atmosphere. \( K_f(\text{water}) = 1.86 \, \text{°C/m} \)
In a laboratory experiment, students synthesized a new compound and found that when 12.93 grams of the compound were dissolved in 273.5 grams of water, the solution began to freeze at -0.488 °C. The compound was also found to be nonvolatile and a non-electrolyte.
What is the molecular weight they determined for this compound?
\[ \_\_\_\_\_\_ \, \text{g/mol} \]
Expert Solution
Step 1
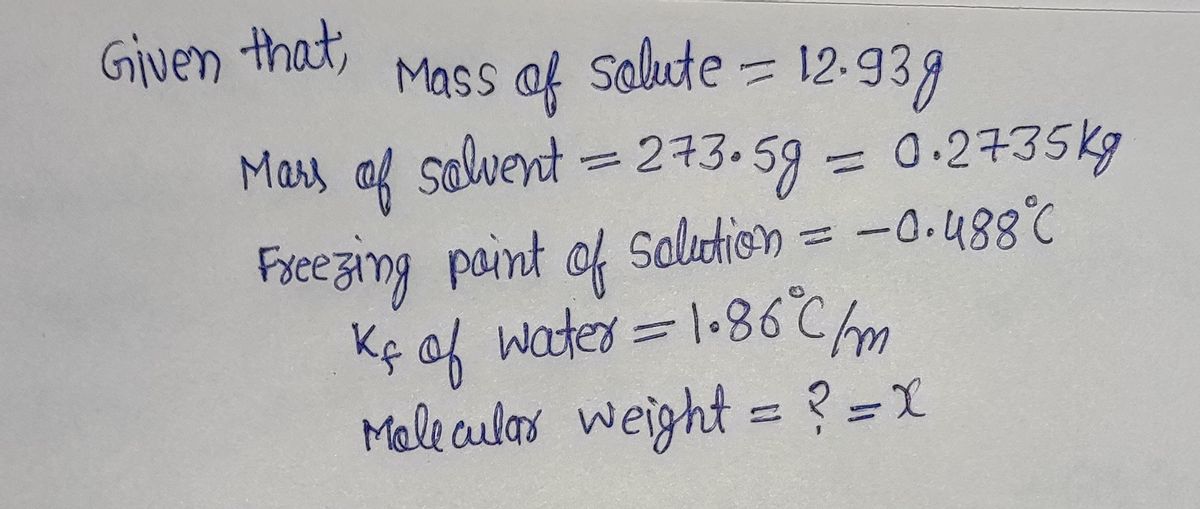
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY