The element zirconium has hcp packing with a hexagonal unit cell. The volume of the unit cell is 4.66 x 10-26 L. Calculate the density (kg/m3) of the element.
The element zirconium has hcp packing with a hexagonal unit cell. The volume of the unit cell is 4.66 x 10-26 L. Calculate the density (kg/m3) of the element.
Hexagonal closed packing refers to layers of spheres packed so that spheres in alternating layers lie over one another. The hexagonal unit cell consists of six atoms per unit cell. The density of the substance is equal to the density of the unit cell. The density of the unit cell can be defined as the ratio of mass of the unit cell to the volume of the unit cell. If d is the density of the unit cell, m is the mass of unit, V is the volume of the unit cell, Z is the number of atoms per unit cell, M is the molar mass of the element and N is the Avogadro number, the density of the unit cell can be written as follows,
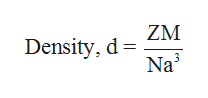
Step by step
Solved in 2 steps with 2 images









