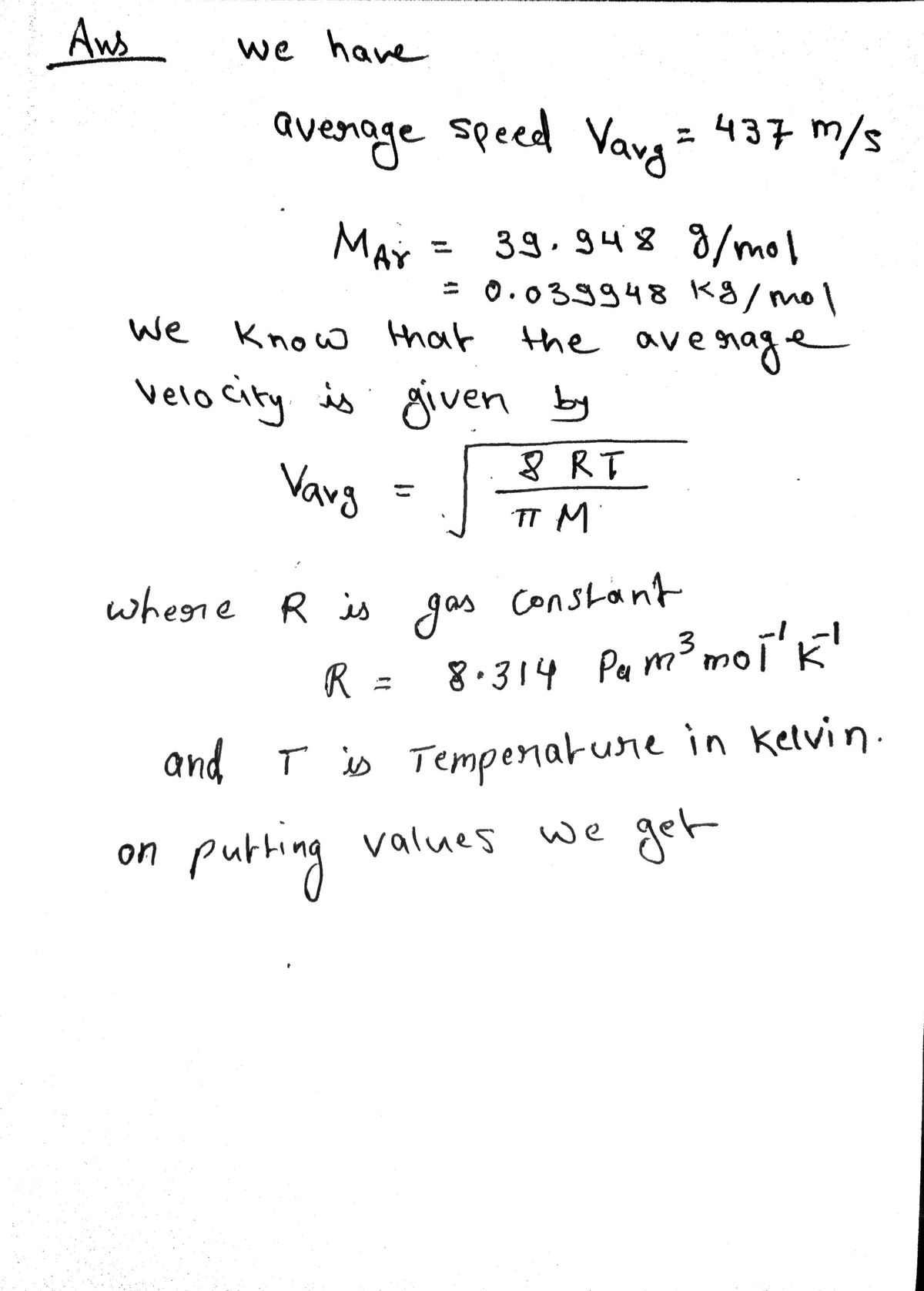
The average speed of a sample of Ar atoms is 437 m/sec. What is the temperature of the sample? (Mar = 39.948 g/mol)
Q: A 2.7 L volume of ideal neon gas (monatomic) is at a pressure of 4.6 atmospheres and a temperature…
A: Given:Initial volume of the gas, V1 = 2.7 LInitial pressure, P1 = 4.6 atmInitial temperature, T1 =…
Q: e enthalpy of Vaporizatio of a liquid is 14.4 KJ at 180K, its boiling point at 1 bar. The molar…
A: Solution: a). Using the Claperon equation the change in vapor pressure can be calculated as,…
Q: A tank of compressed air of volume 1.00 m3 is pressurized to 24.6 atm at T = 273 K. A valve is…
A: V = 1.00m3 Pi = 24.6atm T = 273K Pf = 12.9atm Boltzmann constant (R) = 1.38*10-23J/K
Q: A sealed container holds 0.020 moles of ideal nitrogen (N₂) gas, at a pressure of 1.5 atm and a…
A: The sealed container holds n=0.020 moles of nitrogen gas. Pressure of the gas is P=1.5 atm…
Q: A 7.50 x 10-3-m³ bottle contains 0.0200 kg of oxygen gas, O2, at 77.0 °C. What is the pressure…
A:
Q: A sample of pure gold has a mass of 15.8 g. Calculate the number of moles in the sample and gold…
A:
Q: An apartment has the dimensions 14 m x 7 m x 5 m. The temperature is 30°C and the relative humidity…
A: Convert the temperature from degree Celsius to Kelvin. The saturation pressure of water at 30°C is…
Q: A tank contains 10.5 g of chlorine gas (Cl₂) at a temperature of 80 °C and an absolute pressure of…
A:
Q: tank of compressed air of volume 1.00 m3 is pressurized to 24.6 atm at T = 273 K. A valve is opened,…
A:
Q: Oxygen gas having a volume of 1180 cm3 at 41.7°C and 1.02 x 105 Pa expands until its volume is 1700…
A: Given, Volume V1 = 1180 cm3 = 1180 x 10-6 m3 Temperature T = 41.7C∘ = 41.7+273 =314.7 K Pressure…
Q: soccer ball has an interior volume of 150 cm3 at a pressure of 3.00 atmospheres and a temperature of…
A: Given Volume = 150 cm3 = 150 × 10 -6 m3 temperature T = 23 0 C = 296 K Change in pressure Δ P = P₂…
Q: If 6.07 moles of an ideal gas has a pressure of 8.00 x 10 Pa and a volume of 0.113 m³, what is the…
A:
Q: An apartment has a living room whose dimensions are 2.8 m x 5.2 m x 6.7 m. Assume that the air in…
A: By, ideal gas equation PV = nRT 1.01×105×2.8×5.2×6.7 = n×8.3×(273+21) n = 4037.68 moles
Q: A 5.00-mol sample of oxygen gas is confined to a pressure of 5 atm at a temperature of 27 degrees…
A: Given values: Temperature, T=27 °C Pressure, P=5 atm Molecular mass of oxygen gas, M=32 g
Q: If 6.010 mol of an ideal gas has a pressure of 3.130×105 Pa and a volume of 0.115 m3, what is the…
A:
Q: In an electrochemical cell, a metal anode lost 0.287 g while a total volume of 0.04651 L of hydrogen…
A: The pressure of Hydrogen will be = 765.9 - 23.88 = 742.02 mm / hg = 0.976 atm Nuclear Mass of…
Q: oom whose dimensions are 2.5 m x 5.3 m x 4.3 m. Assume that the air in the room is composed of 79%…
A: Given:- An apartment has a living room whose dimensions are 2.5 m x 5.3 m x 4.3 m. Assume that the…
Q: A tank contains 10.6 g of chlorine gas (Cl₂) at a temperature of 84 °C and an absolute pressure of…
A: Given: Mass m=10.6 g, Molecular weight (mass per mole) M=70.9 g/mol, Temperature T=84°C=(273+84)…
Q: A 5 L tank contains Argon gas at 55°C and 4.5 atm. Find the total translational kinetic energy of…
A: Given data The volume of the gas is V = 5 L = 5 x 10-3 m3 The temperature of the gas is T = 55°C =…
Q: A gas molecule with a molecular mass of 44u has a speed of 337 m/s. What is the temperature of the…
A:
Q: A sealed 73 m tank is filled with 8000 moles of ideal oxygen gas (diatomic) at an initial…
A:
Q: The volume of an automobile tire is 2.5 × 10−2m3 . the pressure of the air in the tire is 3 atm and…
A: According to ideal gas law - Where P = Pressure V = Volume n = Number of moles T = Temperature…
Q: 0 moles of oxygen at a temperature of 150.ºC are confined to a rigid container whose volume is 2.00…
A: Ideal Gas law gives us a relation between temperature and pressure. It states, PV=nRT V is the…

Step by step
Solved in 2 steps with 2 images

- 6. The following data show the relation of vapour pressure of liquid Z as a function of temperature. p/mmHg T/°C 17.54 20 31.82 30 55.32 40 92.51 50 149.38 60 233.70 70 Using linear regression technique, compute the molar enthalpy of vaporization of liquid Z!What is the root-mean- Square Speed in mls of gas molecules with molar molecular weight 33 gimöl at a temperature of 87.29 °C? T=87.29°C + 273 33 gimol 360. 29 k = 0.033 kg/mel2. For T = 300 K, calculate the pressure (in bars) at which the mean free path of a hydrogen molecule will be each of the lengths given here. For H₂, o = 2.30 x 10-1⁹ m². (a) 100 μm (b) 1.00 mm (c) 1.00 m LL
- A sample of argon gas is in a container at 35.0° C and 1.22 atm pressure. The radius of an argon atom (assumed spherical) is 0.710 x 10-10 m. calculate the available volume for each atom.What is the density of water vapor in the air on a hot, dry day in the desert when the temperature is 40.0 °C and the relative humidity is 5.00 %? The vapor pressure of water at 40.0 °C is Py = 7.34 x 10° Pa and the molecular mass of water is M = 18.0 g/mol. Enter your answer in units of grams per cubic meter. density: g/m3The pressure unit 1 torr is comparable to 0.001 bar. In a sample of N₂ at this pressure and a temperature of 300 K, what is the mean free path between collisions in mm (millimeters)? Assume that the cross-section for N₂ molecules is o = 4.50 × 10-19 m².
- 8. The average energy of a mole of Einstein solid is given by: ӨЕ 3R0E Ē = 3R + 2 ӨЕ ет — 1 where E is the Einstein temperature and R the gas constant. Obtain expressions for the average energy at the limits of zero and infinite temperature and comment on your results.3. A spherical balloon is made from a material whose mass is 3.50 kg. The thickness of the material is negligible compared to the 1.30 m radius of the balloon. The balloon is filled with helium (He) at a temperature of 300 K and just floats in air, neither rising nor falling. The density of the surrounding air is 1.19 kg/m3 and the molar mass of helium is 4.0026x10 kg/mol. Find the absolute pressure of the helium gas. Pa 60 ssf6A hot air balloon uses the principle of buoyancy to create lift. By making the air inside the balloon less dense then the surrounding air, the balloon is able to lift objects many times its own weight. A large hot air balloon has a maximum balloon volume of 2090 m3. a. If the air temperature in the balloon is 54 °C, how much additional mass, in kilograms, can the balloon lift? Assume the molar mass of air is 28.97 g/mol, the air density is 1.20 kg/m3, and the air pressure is 1 atm.