System temperature, Tr, where Tcu > Tr> T. According to energy conservation, energy lost by the initially higher temperature of the system will be gained by the initially lower temperature parts of the system Thus the sum of all the gain and loss terms should equal zero. mcuCou(Tf- Tau) + mwCw(Tf- T1) + MAICAI (Tf- T1) = 0 This can be rearranged to find the specific heat of the copper sample: [mwCw(Tr-Ti) + MAICAI (Tr- Ti)]/[mcu(Tcu- T)] CCu = In this experiment, you will determine the specific heat capacity of copper experimentallly, using calorimetry. You will also calculate the associated uncertaint
System temperature, Tr, where Tcu > Tr> T. According to energy conservation, energy lost by the initially higher temperature of the system will be gained by the initially lower temperature parts of the system Thus the sum of all the gain and loss terms should equal zero. mcuCou(Tf- Tau) + mwCw(Tf- T1) + MAICAI (Tf- T1) = 0 This can be rearranged to find the specific heat of the copper sample: [mwCw(Tr-Ti) + MAICAI (Tr- Ti)]/[mcu(Tcu- T)] CCu = In this experiment, you will determine the specific heat capacity of copper experimentallly, using calorimetry. You will also calculate the associated uncertaint
Related questions
Question
Please show me how did they got the rearranged equation because should there be a negative somewhere?
![After thermal equilibrium is reached, the entire system reaches a common
temperature, Tr, where Tcu > Tr > T.
According to energy conservation, energy lost by the initially higher temperature
of the system will be gained by the initially lower temperature parts of the system
Thus the sum of all the gain and loss terms should equal zero.
mcuCCu(Tr- Tcu) + mwCw(Tf- Ti) + MAICAI(Tr - T1) = 0
This can be rearranged to find the specific heat of the copper sample:
Cau = [mwCw(Tr- Ti) + MAICAI(Tr- Ti)]/[mcu(Tcu- T?)]
In this experiment, you will determine the specific heat capacity of copper
experimentally, using calorimetry. You will also calculate the associated uncertainty
the specific heat capacity, using error propagation methods.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fd06dc394-0a4a-49e7-92ae-90f943c8d784%2F9581678b-1a00-406e-b579-65206ebed9ea%2F0a63xsa_processed.jpeg&w=3840&q=75)
Transcribed Image Text:After thermal equilibrium is reached, the entire system reaches a common
temperature, Tr, where Tcu > Tr > T.
According to energy conservation, energy lost by the initially higher temperature
of the system will be gained by the initially lower temperature parts of the system
Thus the sum of all the gain and loss terms should equal zero.
mcuCCu(Tr- Tcu) + mwCw(Tf- Ti) + MAICAI(Tr - T1) = 0
This can be rearranged to find the specific heat of the copper sample:
Cau = [mwCw(Tr- Ti) + MAICAI(Tr- Ti)]/[mcu(Tcu- T?)]
In this experiment, you will determine the specific heat capacity of copper
experimentally, using calorimetry. You will also calculate the associated uncertainty
the specific heat capacity, using error propagation methods.
Expert Solution
Step 1
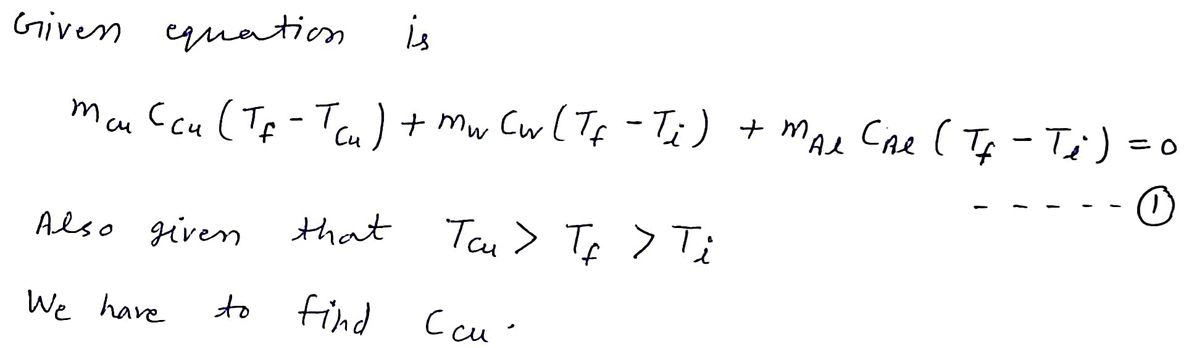
Step by step
Solved in 2 steps with 2 images
