Suppose 75.2 mL of a 0.119 M solution of Na₂SO4 reacts with 165 mL of a 0.383 M solution of MgCl, to produce MgSO, and NaCl as shown in the balanced reaction. Na,SO (aq) + MgCl,(aq) →→ MgSO,(s) + 2NaCl(aq) Determine the limiting reactant for the given reaction. 6 MgSO4 O MgCl₂ NaCl Na₂SO4 Calculate the mass of MgSO4 that can be produced in the given reaction. A mass of MgSO4: Only 0.488 g of MgSO, are isolated after carrying out the reaction. Calculate the percent yield of MgSO4. percent yield: ch or type URL MacBook Pro & 7 * 8 + ( 9 0 I + 11 g %
Suppose 75.2 mL of a 0.119 M solution of Na₂SO4 reacts with 165 mL of a 0.383 M solution of MgCl, to produce MgSO, and NaCl as shown in the balanced reaction. Na,SO (aq) + MgCl,(aq) →→ MgSO,(s) + 2NaCl(aq) Determine the limiting reactant for the given reaction. 6 MgSO4 O MgCl₂ NaCl Na₂SO4 Calculate the mass of MgSO4 that can be produced in the given reaction. A mass of MgSO4: Only 0.488 g of MgSO, are isolated after carrying out the reaction. Calculate the percent yield of MgSO4. percent yield: ch or type URL MacBook Pro & 7 * 8 + ( 9 0 I + 11 g %
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter3: Chemical Reactions
Section: Chapter Questions
Problem 11QRT
Related questions
Question
![### Stoichiometry and Percent Yield in Chemical Reactions
#### Problem Statement
- Suppose 75.2 mL of a 0.119 M solution of Na₂SO₄ reacts with 165 mL of a 0.383 M solution of MgCl₂ to produce MgSO₄ and NaCl as shown in the balanced reaction:
\[
\text{Na}_2\text{SO}_4(\text{aq}) + \text{MgCl}_2(\text{aq}) \rightarrow \text{MgSO}_4(\text{s}) + 2\text{NaCl}(\text{aq})
\]
#### Tasks
1. **Determine the Limiting Reactant:**
- Options:
- MgSO₄
- MgCl₂
- NaCl
- Na₂SO₄ (this option is selected)
2. **Calculate the Mass of MgSO₄ That Can Be Produced:**
- Mass of MgSO₄: \_\_\_\_\_\_\_\_ g
3. **Calculate Percent Yield:**
- Given: Only 0.488 g of MgSO₄ are isolated after carrying out the reaction.
- Calculate the percent yield of MgSO₄:
\[
\text{Percent yield:} \_\_\_\_\_\_\_\_ \%
\]
---
#### Explanation
**Balanced Reaction:**
The stoichiometry of the reaction indicates a 1:1 molar ratio between Na₂SO₄ and MgCl₂ to produce one mole of MgSO₄ and two moles of NaCl.
**Limiting Reactant:**
The limiting reactant is the substance that will be completely consumed first during the reaction, thus limiting the amount of product formed. Based on the given molarity and volumes, the correct choice is Na₂SO₄.
**Mass Calculation:**
To calculate the mass of MgSO₄ produced:
- Calculate moles of reactants.
- Determine the limiting reactant.
- Use the stoichiometric relationship to find moles of MgSO₄.
- Convert moles of MgSO₄ to grams (using molar mass).
**Percent Yield Calculation:**
The percent yield is calculated as follows:
\[
\text{Percent Yield} = \left( \frac{\text{Actual Yield}}{\text{Theoretical Yield](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fa488a3d5-fb60-4389-a69a-9fdfd1297bd5%2Ff9a36e7a-271b-4236-9f94-40327819b027%2Fmfoh6dv_processed.jpeg&w=3840&q=75)
Transcribed Image Text:### Stoichiometry and Percent Yield in Chemical Reactions
#### Problem Statement
- Suppose 75.2 mL of a 0.119 M solution of Na₂SO₄ reacts with 165 mL of a 0.383 M solution of MgCl₂ to produce MgSO₄ and NaCl as shown in the balanced reaction:
\[
\text{Na}_2\text{SO}_4(\text{aq}) + \text{MgCl}_2(\text{aq}) \rightarrow \text{MgSO}_4(\text{s}) + 2\text{NaCl}(\text{aq})
\]
#### Tasks
1. **Determine the Limiting Reactant:**
- Options:
- MgSO₄
- MgCl₂
- NaCl
- Na₂SO₄ (this option is selected)
2. **Calculate the Mass of MgSO₄ That Can Be Produced:**
- Mass of MgSO₄: \_\_\_\_\_\_\_\_ g
3. **Calculate Percent Yield:**
- Given: Only 0.488 g of MgSO₄ are isolated after carrying out the reaction.
- Calculate the percent yield of MgSO₄:
\[
\text{Percent yield:} \_\_\_\_\_\_\_\_ \%
\]
---
#### Explanation
**Balanced Reaction:**
The stoichiometry of the reaction indicates a 1:1 molar ratio between Na₂SO₄ and MgCl₂ to produce one mole of MgSO₄ and two moles of NaCl.
**Limiting Reactant:**
The limiting reactant is the substance that will be completely consumed first during the reaction, thus limiting the amount of product formed. Based on the given molarity and volumes, the correct choice is Na₂SO₄.
**Mass Calculation:**
To calculate the mass of MgSO₄ produced:
- Calculate moles of reactants.
- Determine the limiting reactant.
- Use the stoichiometric relationship to find moles of MgSO₄.
- Convert moles of MgSO₄ to grams (using molar mass).
**Percent Yield Calculation:**
The percent yield is calculated as follows:
\[
\text{Percent Yield} = \left( \frac{\text{Actual Yield}}{\text{Theoretical Yield
Expert Solution
Step 1
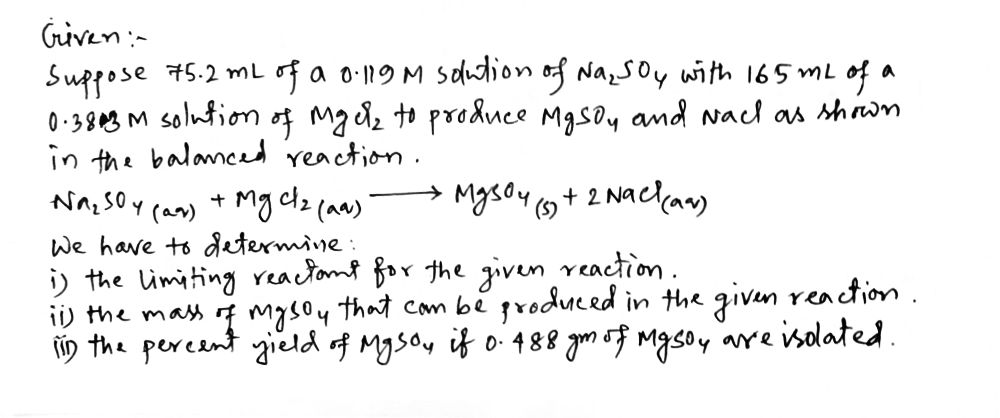
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning