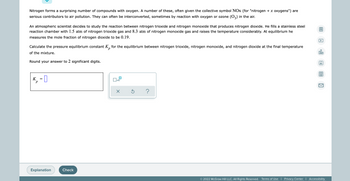
Ethylene (CH₂CH₂) is the starting point for a wide array of industrial chemical syntheses. For example, worldwide about 8.0 × 10¹0 kg of polyethylene are made from ethylene each year, for use in everything from household plumbing to artificial joints. Natural sources of ethylene are entirely inadequate to meet world demand, so ethane (CH3 CH3) from natural gas is "cracked" in refineries at high temperature in a kinetically complex reaction that produces ethylene gas and hydrogen gas. 圖 Suppose an engineer studying ethane cracking fills a 50.0 L reaction tank with 20.0 atm of ethane gas and raises the temperature to 600. °C. She believes Kp = 0.70 at this temperature. dlo Calculate the percent by mass of ethylene the engineer expects to find in the equilibrium gas mixture. Round your answer to 2 significant digits. Ar Note for advanced students: the engineer may be mistaken about the correct value of Kp, and the mass percent of ethylene you calculate may not be what she actually observes. 99 % X 5 ? Explanation Check
Thermochemistry
Thermochemistry can be considered as a branch of thermodynamics that deals with the connections between warmth, work, and various types of energy, formed because of different synthetic and actual cycles. Thermochemistry describes the energy changes that occur as a result of reactions or chemical changes in a substance.
Exergonic Reaction
The term exergonic is derived from the Greek word in which ‘ergon’ means work and exergonic means ‘work outside’. Exergonic reactions releases work energy. Exergonic reactions are different from exothermic reactions, the one that releases only heat energy during the course of the reaction. So, exothermic reaction is one type of exergonic reaction. Exergonic reaction releases work energy in different forms like heat, light or sound. For example, a glow stick releases light making that an exergonic reaction and not an exothermic reaction since no heat is released. Even endothermic reactions at very high temperature are exergonic.

Step by step
Solved in 4 steps with 3 images

this one is asking for kg
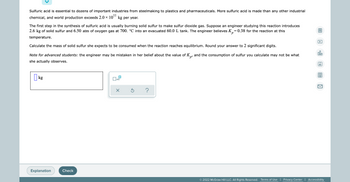
This one is a little different, but can u answer it as well