Students at Gonzaga University were attempting to make barium phosphate by precipitation when mixing solutions of barium chloride and trisodium phosphate: 17 3 BaCl₂ (aq) + 2 Na3PO(aq) → Ba3(PO4)₂(s) + 6 NaCl(aq) FM 208.23 FM 163.94 FM 601.92 In one case, 0.479 g of BaCl, and 0.667 g of Na3PO4 were each dissolved separately in ~50 mL H₂O in beakers. The Na3PO4 solution was poured into the BaCl₂ solution with mixing and the resulting suspension of white powder was digested at 80°C for 30 min to give 0.766 g of white solid after filtration, washing, and air drying.
Students at Gonzaga University were attempting to make barium phosphate by precipitation when mixing solutions of barium chloride and trisodium phosphate: 17 3 BaCl₂ (aq) + 2 Na3PO(aq) → Ba3(PO4)₂(s) + 6 NaCl(aq) FM 208.23 FM 163.94 FM 601.92 In one case, 0.479 g of BaCl, and 0.667 g of Na3PO4 were each dissolved separately in ~50 mL H₂O in beakers. The Na3PO4 solution was poured into the BaCl₂ solution with mixing and the resulting suspension of white powder was digested at 80°C for 30 min to give 0.766 g of white solid after filtration, washing, and air drying.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:Students at Gonzaga University were attempting to make barium phosphate by precipitation when mixing solutions of barium
chloride and trisodium phosphate: 17
3 BaCl₂ (aq) + 2 Na3PO(aq) → Ba3(PO4)₂(s) + 6 NaCl(aq)
FM 208.23
FM 163.94
FM 601.92
In one case, 0.479 g of BaCl₂ and 0.667 g of Na3PO4 were each dissolved separately in ~50 mL H₂O in beakers. The Na3PO4
solution was poured into the BaCl₂ solution with mixing and the resulting suspension of white powder was digested at 80°C for
30 min to give 0.766 g of white solid after filtration, washing, and air drying.

Transcribed Image Text:Use the gravimetric factor to calculate theoretical yield.
theoretical yield:
0.9229
observed yield:
Incorrect
What is the observed yield (%)?
82.99
Incorrect
g
%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
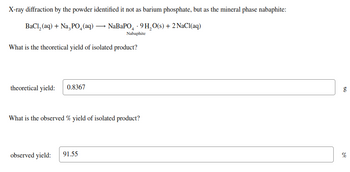
Transcribed Image Text:X-ray diffraction by the powder identified it not as barium phosphate, but as the mineral phase nabaphite:
BaCl,(aq) + NaşPO_(aq) — NaBaPO - 9H,O(s)+2NaCl(aq)
Nabaphite
What is the theoretical yield of isolated product?
theoretical yield: 0.8367
What is the observed % yield of isolated product?
observed yield:
91.55
g
%

Transcribed Image Text:When the same procedure was conducted, but digestion was carried out in a sealed Teflon bomb at 105°C for 3 h instead of
80°C for 30 min, 0.361 g of white solid product was collected and identified by X-ray diffraction as Ba, (PO4)₂, not nabaphite.
What was the % yield of this product?
oberved yield:
39.11
%
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY