Stable molecules form when atoms arrange in a way to exert equal force on each other. One such molecule is phosphate, PO³-, which forms a tetrahedron shape. The notation indicates 4 oxygen atoms with a single phosphorus atom (and an overall excess of 3 electrons). Consider a coordinate system where the four oxygen atoms that form the tetrahedron are placed at (1, 0, 0), (0, 1, 0), (0, 0, 1), and (−1/3, -1/3, -1/3). (a) To have equal forces the phosphate atom must be placed equidistant from each of the oxygen atoms. Assume the phosphorus atom has the same value for all coordinates, i.e. it is located at (a, a, a). Find a. (b) Determine the bond angle, that is the angle between the lines that join the phosphorus atom to two of the oxygen atoms. Estimate it in degrees.
Stable molecules form when atoms arrange in a way to exert equal force on each other. One such molecule is phosphate, PO³-, which forms a tetrahedron shape. The notation indicates 4 oxygen atoms with a single phosphorus atom (and an overall excess of 3 electrons). Consider a coordinate system where the four oxygen atoms that form the tetrahedron are placed at (1, 0, 0), (0, 1, 0), (0, 0, 1), and (−1/3, -1/3, -1/3). (a) To have equal forces the phosphate atom must be placed equidistant from each of the oxygen atoms. Assume the phosphorus atom has the same value for all coordinates, i.e. it is located at (a, a, a). Find a. (b) Determine the bond angle, that is the angle between the lines that join the phosphorus atom to two of the oxygen atoms. Estimate it in degrees.
Advanced Engineering Mathematics
10th Edition
ISBN:9780470458365
Author:Erwin Kreyszig
Publisher:Erwin Kreyszig
Chapter2: Second-order Linear Odes
Section: Chapter Questions
Problem 1RQ
Related questions
Question

Transcribed Image Text:Stable molecules form when atoms arrange in a way to exert equal force on each other. One
such molecule is phosphate, PO³-, which forms a tetrahedron shape. The notation indicates 4
oxygen atoms with a single phosphorus atom (and an overall excess of 3 electrons). Consider
a coordinate system where the four oxygen atoms that form the tetrahedron are placed at
(1, 0, 0), (0, 1, 0), (0, 0, 1), and (−1/3, -1/3, -1/3).
(a) To have equal forces the phosphate atom must be placed equidistant from each of the
oxygen atoms. Assume the phosphorus atom has the same value for all coordinates, i.e.
it is located at (a, a, a). Find a.
(b) Determine the bond angle, that is the angle between the lines that join the phosphorus
atom to two of the oxygen atoms. Estimate it in degrees.
Expert Solution
Step 1
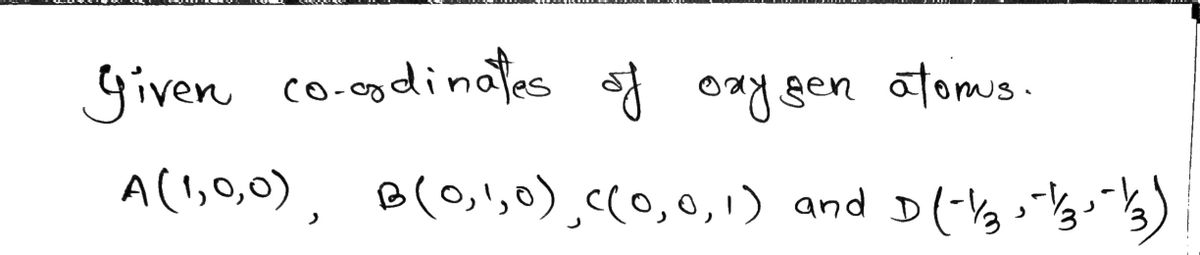
Step by step
Solved in 3 steps with 5 images

Recommended textbooks for you

Advanced Engineering Mathematics
Advanced Math
ISBN:
9780470458365
Author:
Erwin Kreyszig
Publisher:
Wiley, John & Sons, Incorporated

Numerical Methods for Engineers
Advanced Math
ISBN:
9780073397924
Author:
Steven C. Chapra Dr., Raymond P. Canale
Publisher:
McGraw-Hill Education

Introductory Mathematics for Engineering Applicat…
Advanced Math
ISBN:
9781118141809
Author:
Nathan Klingbeil
Publisher:
WILEY

Advanced Engineering Mathematics
Advanced Math
ISBN:
9780470458365
Author:
Erwin Kreyszig
Publisher:
Wiley, John & Sons, Incorporated

Numerical Methods for Engineers
Advanced Math
ISBN:
9780073397924
Author:
Steven C. Chapra Dr., Raymond P. Canale
Publisher:
McGraw-Hill Education

Introductory Mathematics for Engineering Applicat…
Advanced Math
ISBN:
9781118141809
Author:
Nathan Klingbeil
Publisher:
WILEY

Mathematics For Machine Technology
Advanced Math
ISBN:
9781337798310
Author:
Peterson, John.
Publisher:
Cengage Learning,

