Solubility of Ionic Compounds Classify each of the compounds as soluble or not soluble: iron(II) iodide magnesium sulfate sodium nitrate soluble not soluble
Ionic Equilibrium
Chemical equilibrium and ionic equilibrium are two major concepts in chemistry. Ionic equilibrium deals with the equilibrium involved in an ionization process while chemical equilibrium deals with the equilibrium during a chemical change. Ionic equilibrium is established between the ions and unionized species in a system. Understanding the concept of ionic equilibrium is very important to answer the questions related to certain chemical reactions in chemistry.
Arrhenius Acid
Arrhenius acid act as a good electrolyte as it dissociates to its respective ions in the aqueous solutions. Keeping it similar to the general acid properties, Arrhenius acid also neutralizes bases and turns litmus paper into red.
Bronsted Lowry Base In Inorganic Chemistry
Bronsted-Lowry base in inorganic chemistry is any chemical substance that can accept a proton from the other chemical substance it is reacting with.
![**Solubility of Ionic Compounds**
Classify each of the compounds as **soluble** or **not soluble**:
1. **iron(II) iodide**
- [Dropdown menu option selected: soluble]
2. **magnesium sulfate**
3. **sodium nitrate**
In this section, users are prompted to classify various ionic compounds based on their solubility. A dropdown menu is provided for each compound, where users can select whether the compound is soluble or not soluble. The example shown has "soluble" selected for iron(II) iodide.
No graphs or diagrams are present in this image.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F0711daf6-3b19-448b-99c0-3e7fe08f4fe3%2Fd1f83a18-b59c-4ca8-b132-05182129e926%2Fyi4b5wm_processed.jpeg&w=3840&q=75)
![**Question:**
A 17.38 gram sample of manganese is heated in the presence of excess chlorine. A metal chloride is formed with a mass of 39.82 g. Determine the empirical formula of the metal chloride.
**Instructions:**
Enter the elements in the order Mn, Cl.
**Answer:**
Empirical formula = [ ]
---
**Explanation:**
To find the empirical formula, determine the moles of manganese and chlorine involved, then calculate the simplest whole number ratio. Fill in the box with the empirical formula in the specified order.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F0711daf6-3b19-448b-99c0-3e7fe08f4fe3%2Fd1f83a18-b59c-4ca8-b132-05182129e926%2Fy5abz4l_processed.jpeg&w=3840&q=75)
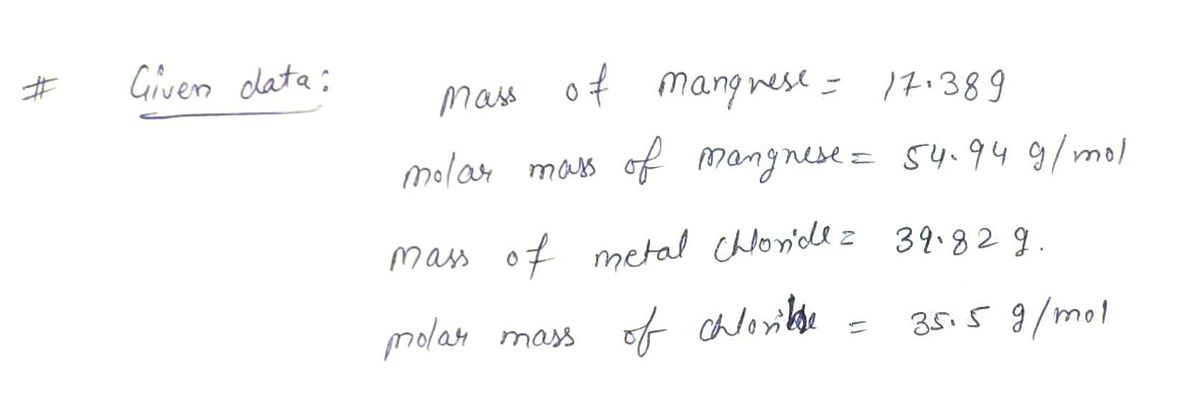
Step by step
Solved in 3 steps with 3 images









