Solid metals can be modeled as a set of uncoupled harmonic oscillators of the same frequency with energy levels given by En = hwn = 0, 1, 2, ... where the zero-point energy (the lowest energy state) of each oscillator has been adjusted to zero for simplicity. In this model, the harmonic oscillators represent the motions of the metal atoms relative to one another. The frequency of these oscillators is low so that ✪ n = ħw KB and the system vibrational partition function is given by 3N = = 224 1 (1 - e O/T (a) If the system contains one mole of atoms, find the average energy (in J) of this system at Ⓒ T= 172 K. (You can use = = pkB.) T (b) What is the absolute entropy (in J/K) for this system? You can use either the Gibbs expression for S, or the system partition function to make this evaluation (they are equivalent, as your reading assignment indicates).
Solid metals can be modeled as a set of uncoupled harmonic oscillators of the same frequency with energy levels given by En = hwn = 0, 1, 2, ... where the zero-point energy (the lowest energy state) of each oscillator has been adjusted to zero for simplicity. In this model, the harmonic oscillators represent the motions of the metal atoms relative to one another. The frequency of these oscillators is low so that ✪ n = ħw KB and the system vibrational partition function is given by 3N = = 224 1 (1 - e O/T (a) If the system contains one mole of atoms, find the average energy (in J) of this system at Ⓒ T= 172 K. (You can use = = pkB.) T (b) What is the absolute entropy (in J/K) for this system? You can use either the Gibbs expression for S, or the system partition function to make this evaluation (they are equivalent, as your reading assignment indicates).
Related questions
Question

Transcribed Image Text:Solid metals can be modeled as a set of uncoupled harmonic oscillators of the same
frequency with energy levels given by
En = ħwn
n = 0, 1, 2,...
where the zero-point energy (the lowest energy state) of each oscillator has been adjusted
to zero for simplicity. In this model, the harmonic oscillators represent the motions of the
metal atoms relative to one another. The frequency of these oscillators is low so that
ħw
= = 224
KB
and the system vibrational partition function is given by
3N
Z
² = la₁ -
(1
1
e-0/T).
(a) If the system contains one mole of atoms, find the average energy (in J) of this system at
T= 172 K. (You can use =
BkB.)
T
(b) What is the absolute entropy (in J/K) for this system? You can use either the Gibbs
expression for S, or the system partition function to make this evaluation (they are
equivalent, as your reading assignment indicates).
Expert Solution
Step 1
The required solution is following
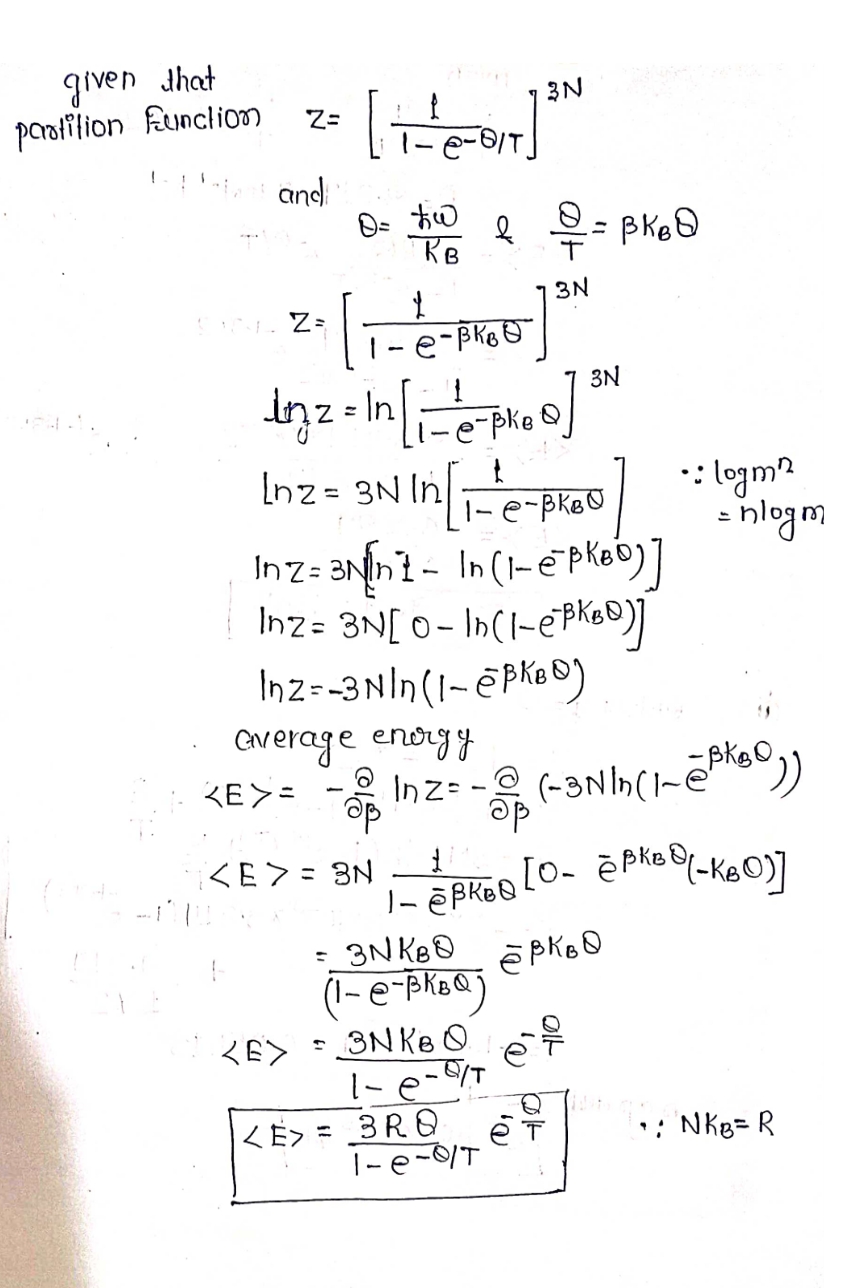
Step by step
Solved in 2 steps with 2 images
