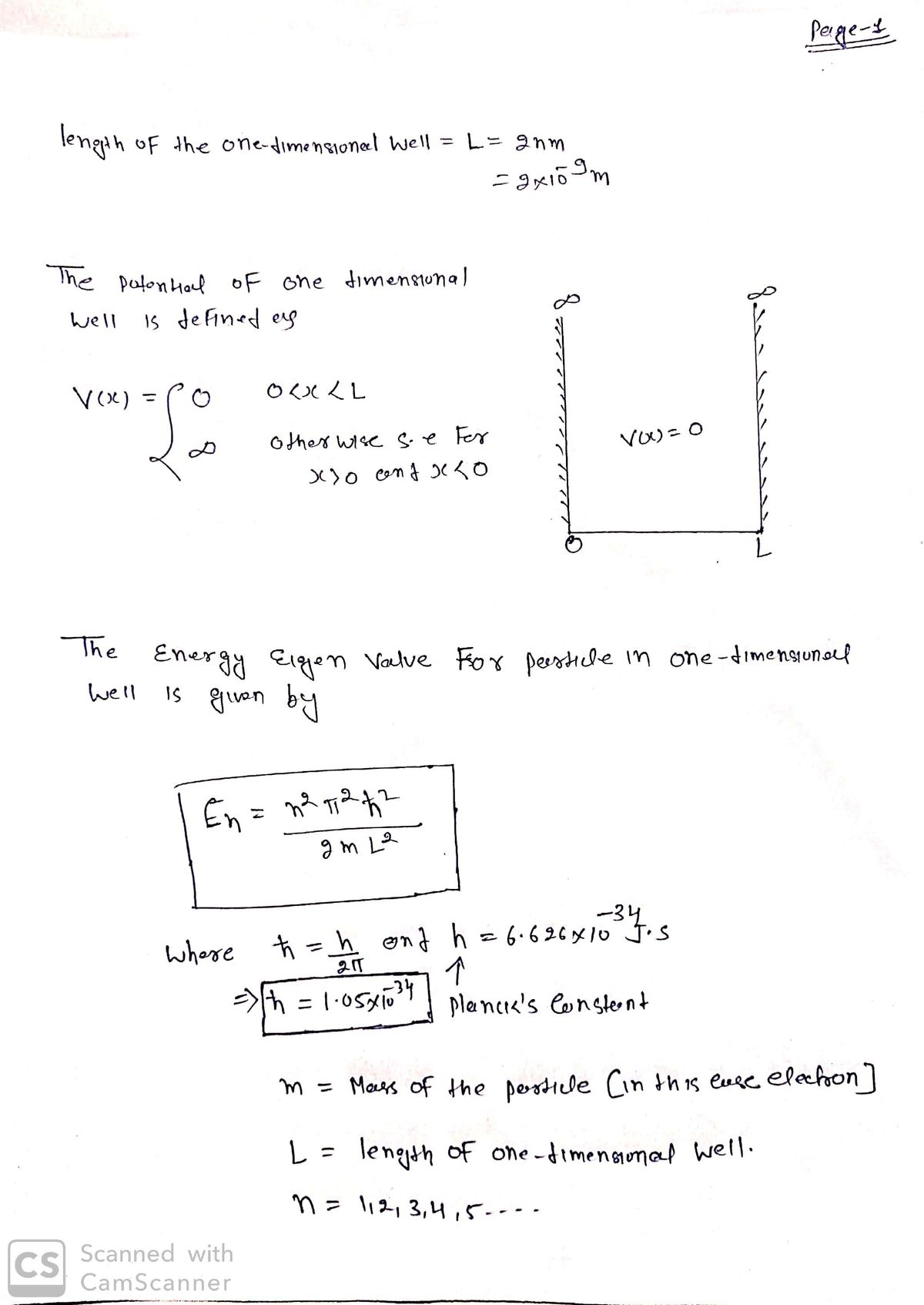
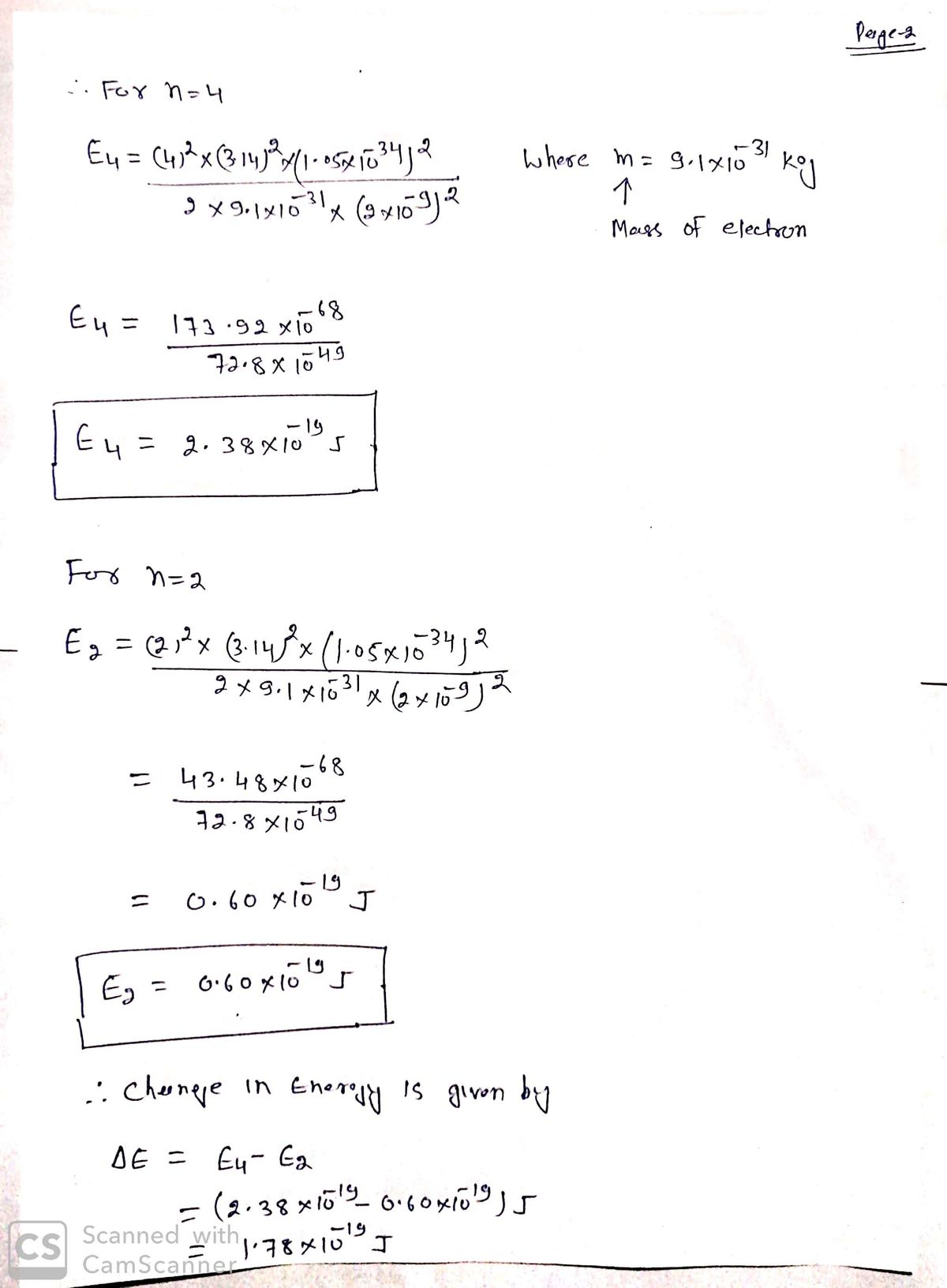
An electron is in a one dimensional well of length L=2nm, with zero potential inside and infinite potential at the walls. If the electron jumps from energy level n=4 to level n=2 what is the frequency of light emitted? Group of answer choices 3.4 x1015 Hz 6.8 x1014 Hz 3.39 x1014 Hz none of the above 6.8 x1013 Hz
Q: An atom has three energy states: -15 eV, -12 eV and -6 eV. If a beam of photons with photons of…
A: Required : The correct option.
Q: An electron orbiting a hydrogen atom transitions from n=6 to n=2. What is the wavelength of the…
A: “Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: As an electron in a one-dimensional box of length 0.600 nm jumps between two energy levels, a photon…
A: Given: The length of box is 0.600 nm. The energy of 8.36 eV.
Q: What is the second level energy of an electron (m=9.11x10-31kg) that is confined to a space of…
A: The second level energy of the electron. given, mass of electron(m)=9.1×10-31 kg Energy level(n)= 2…
Q: a. An electron is found to a wavelength of 1 = 1.19 nm. What is the velocity of the electron? m/s b.…
A:
Q: beam of electrons is accelerated across a potential of 14.40 kV before passing through two slits.…
A:
Q: two one-dimensional boxes (Box A and Box B) each contain one electron (mass: 9.11*10^-31 kg). Box B…
A: The energy required to excite an electron in Box B from the ground state to the n = 6 state can be…
Q: A/What is the wavelength of an electron with a kinetic energy of 49.8 eV? (Possibly useful…
A:
Q: Electrons are ejected from a metal surface with the speeds ranging up to v=4.6x10° m/s when light…
A:
Q: An electron is trapped in a is absorbed, the electron is in the n = 6 energy level. What was the…
A:
Q: In an advanced laboratory class a student performs the photoelectric experiment. Ultraviolet light…
A: Stopping potential for wavelength is Stopping potential for is Note:Speed of light is The…
Q: a. An electron is found to a wavelength of A = 0.65 nm. What is the velocity of the electron? m/s b.…
A: (a) Using de-Broglie wavelength equation: λ=hmvv=hmλ where,…
Q: Electrons, thermionically emitted from a cathode in a vacuum valve, travel across a potential…
A: Relation between kinetic energy and stopping potential is KE = eV e is charge of electron but…
Q: Find the de Broglie wavelength of a proton (m = 1.67 x 10-27 kg) moving with a speed of 1.11 x 107…
A:
Q: The mass of an electron is about 9.11x10^-31 kg. What is the de Broglie wavlength of an electron…
A: Given data: The mass of an electron (m) = 9.11×10-31 kg Speed (v) = 0.01 m/s Required: The de…
Q: (b) What is the cutoff frequency for this surface?
A:
Q: In an experiment designed to measure the charge-to-mass ratio of the electron, electrons are…
A:
Q: Calculate the de Broglie wavelength for an electron that has kinetic energy 48.4eV B. Calculate the…
A: Given: E1=48.4 eV = 48.4*1.6*10-19JE1=7.74*10-18JandE2=48.4 KeV = 48.4*103*1.6*10-19 JE2=7.74*10-15…
Q: 4C. Electrons in one electron beam have a greater speed than those in another. Which electrons have…
A: Here's the formula for the de Broglie wavelength (λ):λ = h / pwhere:λ (lambda) is the de Broglie…
Q: A proton and an electron are accelerated from rest through the same potential difference V. What is…
A:
Q: Comment Edit Page Protect Tools 1. The photoelectric threshold wavelength of a tungsten surface is…
A:
Q: A. Electrons are ejected from a metal surface with speeds ranging up to 4.72E+5m/s when light with…
A: Given: The speed of the electron is 4.72x105 m/s. The wavelength of the light is 650 nm.
Q: The figure shows a model of the energy levels of an atom. The atom is initially in state W, which is…
A: When an electron goes from lower state to higher energy state, it requires energy. Therefore, for…
Q: Electrons with a speed of 2.0 x 106 m/s pass through a double-slit apparatus. Interference fringes…
A: (a) The wave length associated with the motion of electron is given by, λe=hmeν Now as we know that…
Q: So Determine the distance between the electron and proton in an atom if the potential energy ?U of…
A: Given, Potential energy of electron proton in an atom, U = 15.4 eV 1 eV = 1.6×10-19 C
Q: An electron and a 0.0440-kg bullet each have a velocity of magnitude 480 m/s, accurate to within…
A:
Q: 5.40x106 atoms are excited to an upper energy level at t = 0 s. At the end of 30.0 ns , 90.0% of…
A: According to question we have to find--- How many photons have been emitted? What is the lifetime…
Q: Problem 1: Light of frequency 9.95 x 1014 Hz ejects electrons from the surface of a metal. If the…
A:
Q: A proton is in a one-dimensional box of width 7.8 pm (1 pm = 1 x 10-¹2 m). The energy of the proton…
A: One proton is in a one dimensional box.Width of box is 7.8 pm Energy of photon = energy of ground…
Q: 1. A magnesium surface has a work function of 3.10 eV. Electromagnetic waves with a wavelength of…
A:
Q: Electrons striking the back of a conventional TV screen travel at a speed of about 1.5 x 107 m/s.…
A: Formula
Q: An electron is moving with a speed of 3.00 x 10° m/s. What is the speed of a proton that has the…
A: We have to use de broglei formula here.
Q: A hydrogen atom (mass= 1.672 x 10-27 kg) quantum jumps from the fourth excited state to the first…
A:
An electron is in a one dimensional well of length L=2nm, with zero potential inside and infinite potential at the walls. If the electron jumps from energy level n=4 to level n=2 what is the frequency of light emitted?
3.4 x1015 Hz
6.8 x1014 Hz
3.39 x1014 Hz
6.8 x1013 Hz
Step by step
Solved in 3 steps with 3 images

- If the wavelength of an electron is 4.58 ✕ 10−7 m, how fast is it moving? km/s(b) If the electron has a speed equal to 3.80 ✕ 106 m/s, what is its wavelength? mThe nucleus of a typical atom is 5.0 fm (1 fm = 10-15 m) in diameter. A very simple model of the nucleus is a onedimensional box in which protons are confined. Estimate the energy of a proton in the nucleus by finding the first three allowed energies of a proton in a 5.0-fm-long box.Electrons striking the back of a conventional TV screen travel at a speed of about 2.7 ✕ 107 m/s. What is their de Broglie wavelength (in nm)?
- A particle of matter is moving with a kinetic energy of 7.53 eV. Its de Broglie wavelength is 2.85 x 10^-12 m. What is the mass of the particle?In a TV tube an electric potential difference accelerates electrons from a rest position towards a screen. Just before reaching the screen, the electrons have a wavelength of 1.2×10−11m1.2×10-11m. Determine the kinetic energy of the electrons just before they reach the screen. Your final answer should have units of eV.An X-ray photon has a wavelength of 0.01 nm. What is the de Broglie wavelength of an electron that has the same energy as the X-ray photon? O 1.5 X 10-3 nm O 3.0 X 10-3 nm O 3.5 X 10-3 nm O 4.0 X 10-3 nm O 2.0 X 10-3 nm O 2.5 X 10-3 nm
- The work function of a metal is is 2.04 e V. What will be the frequency of light required to eject electrons? * (2 Points) 3.2 x10 ^15 Hz O 4.2 x10 ^15 Hz O 3.2 x10 ^15 Hz 4.9 x19 ^14 HzDuring a certain experiment, the de Broglie wavelength of an electron is 610 nm = 6.1 x 10- m, which is the same as the wavelength of orange light. How fast (in m/s) is the electron moving? m/s