Shown below is information about a voltaic cell. Cr | Cr**(.75 M) || Ni*(?) | Niº a. Write the oxidation and reduction half reactions. Indicate if the half reaction is occurring at the cathode or anode. Then calculate the cell potential at standard state conditions and write the net redox reaction. b. If the Ecel produced by the above cell was found to be 0.45 V, what is the concentration of nickel(II) ion? The temperature is 20°C.
![### Voltaic Cell Information
#### Problem 10
The information given pertains to a voltaic cell described as follows:
\[ \text{Cr}^0 \mid \text{Cr}^{3+} (0.75 \, \text{M}) \parallel \text{Ni}^{2+} (?) \mid \text{Ni}^0 \]
##### Part (a)
**Task:**
1. **Write the oxidation and reduction half reactions.** Indicate if the half reaction is occurring at the cathode or anode.
2. **Calculate the cell potential** at standard state conditions.
3. Write the **net redox reaction**.
##### Part (b)
**Task:**
- Given that the \(E_{\text{cell}}\) produced by the above cell was found to be 0.45 V, **determine the concentration of the nickel(II) ion** (\(\text{Ni}^{2+}\)). The temperature is given as 20°C.
---
#### Detailed Analysis and Solutions
**Half Reactions Identification:**
- The half reactions are essential to understand how the electrons are transferred in the redox reaction.
- In each half reaction:
- **Oxidation**: Occurs at the anode.
- **Reduction**: Occurs at the cathode.
**Calculation of Cell Potential:**
- The cell potential (\(E_{\text{cell}}\)) at standard state conditions can be calculated using the standard reduction potentials of the given species.
**Net Redox Reaction:**
- Combine the oxidation and reduction half reactions to form a balanced net ionic equation.
**Concentration Calculation:**
- Using the Nernst equation:
\[
E_{\text{cell}} = E_{\text{cell}}^\circ - \frac{RT}{nF} \ln Q
\]
where \(E_{\text{cell}}\) is the measured cell potential, \(E_{\text{cell}}^\circ\) is the standard cell potential, \(R\) is the gas constant, \(T\) is the temperature in Kelvin, \(n\) is the number of moles of electrons transferred, \(F\) is the Faraday constant, and \(Q\) is the reaction quotient.
**Given:**
- Temperature: 20°C = 293.15 K
- Measured cell potential \(E_{\text{cell}}\): 0](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F3cf64a17-58e5-4321-8fe0-e348a1cc4f42%2F06c9c48a-ff79-4c90-ae4f-4e672a024e71%2Fgl8nvgm.png&w=3840&q=75)
Expression consisting all cell's ions of any electrochemical as well as electrolytic cell is identified as "Nernst equation". This creates an relationship among various reaction factors such as electrons involved, temperature and concentration.
Given:
The cell potential is 0.45 V.
The concentration of chromium ions is 0.75 M.
The temperature is 20 degree Celsius.
Part (a):
In the given cell, chromium undergoes oxidation at anode and nickel undergoes reduction at cathode.
The oxidation half-reaction at anode is shown below.
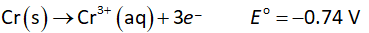
The reduction half-reaction at cathode is shown below.
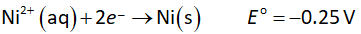
Multiply oxidation half-reaction with 2 and reduction half-reaction with 3 to balance the electrons transferred (n=3).
Then, add oxidation and reduction half-reactions to obtain net reaction as shown below.
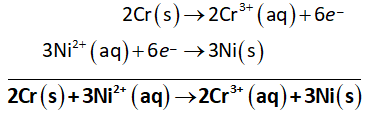
The formula to calculate standard cell potential is shown below.
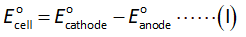
Here,
The electrode potential of cathode is -0.25 V.
The electrode potential of anode is -0.74 V.
Substitute the required values in equation (I).
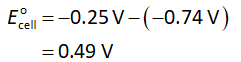
Step by step
Solved in 6 steps with 11 images









