Seven thousand lb/h of wet, pulverized clay particles with 27% moisture (dry basis) at 15oC and 1 atm enter a flash dryer, where they are dried to a moisture content of 5% (dry basis) with a cocurrent flow of air that enters at 525oC. The dried solids exit at the air wet-bulb temperature of 50oC, while the air exits at 75oC. Assuming the specific heat of clay=0.3 Btu/lb-oF, calculate: (a) flow rate of air in lb/h (dry basis); (b) rate of evaporation of moisture; (c) heattransfer rate in Btu/h.
Seven thousand lb/h of wet, pulverized clay particles with 27% moisture (dry basis) at 15oC and 1 atm enter a flash dryer, where they are dried to a moisture content of 5% (dry basis) with a cocurrent flow of air that enters at 525oC. The dried solids exit at the air wet-bulb temperature of 50oC, while the air exits at 75oC. Assuming the specific heat of clay=0.3 Btu/lb-oF, calculate: (a) flow rate of air in lb/h (dry basis); (b) rate of evaporation of moisture; (c) heattransfer rate in Btu/h.
The solution to part (a) is linked with answers of part (b) and part (c). So, part (a) must be solved at last.
(b) The moisture evaporation rate for the particles is to be determined.
Consider the following notations:-
F = Feed flow rate
m1 = Mass flow rate of clay
m2 = Mass flow rate of the moisture
EM = Moisture evaporated in dryer
X = Initial moisture present
X' = Final moisture present
Given,
F = 7000 lb/h
X = 0.27
X' = 0.05
Thus,
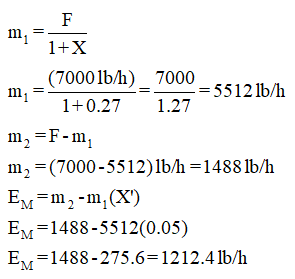
(c) The rate of transfer of heat for the given process is to be determined.
The heat transfer rate is given as,
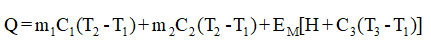 ....... (1)
....... (1)
The notations used in equation (1) are:-
Q = Rate of heat transfer
C1 = Specific heat of clay
C2 = Specific heat of moisture
C3 = Specific heat of evaporated substance
H = Heat of vaporization at 500C
T1 = Temperature at the entrance for clay particles
T2 = Wet bulb temperature
T3 = Temperature at the exit
Step by step
Solved in 10 steps with 7 images









