See if you can complete the following table using only what is on this page. Hint: Two of the entries are for the same element. Element Nuclear Atomic Mass Protons Neutrons Electrons Symbol Symbol Number Number Be 17 20
See if you can complete the following table using only what is on this page. Hint: Two of the entries are for the same element. Element Nuclear Atomic Mass Protons Neutrons Electrons Symbol Symbol Number Number Be 17 20
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:The image contains a table meant to test knowledge of atomic structure. It includes several columns to be filled out based on given information. Here is the content of the table with explanations:
### Table Columns:
1. **Element Symbol**
2. **Nuclear Symbol**
3. **Atomic Number**
4. **Mass Number**
5. **Protons**
6. **Neutrons**
7. **Electrons**
### Provided Data:
- **First Row:**
- **Element Symbol:** \(^{35}_{17}\)Cl
- **Neutrons:** Not provided
- **Electrons:** Not provided
- **Second Row:**
- **Element Symbol:** Be
- **Nuclear Symbol:** Not provided
- **Atomic Number:** 8
- **Mass Number:** Not provided
- **Protons:** Not provided
- **Electrons:** 4
- **Third Row:**
- **Element Symbol:** Not provided
- **Nuclear Symbol:** Not provided
- **Atomic Number:** Not provided
- **Mass Number:** Not provided
- **Protons:** 17
- **Neutrons:** 20
- **Electrons:** Not provided
### Instructions:
Students are instructed to complete the table using only the given information and hints. Two entries are for the same element, which serves as a clue to fill in the missing data.
### Submission:
Below the table are buttons for "Submit Answer" and "Retry Entire Group", with a note indicating there are 7 more group attempts remaining.
Expert Solution
Step 1
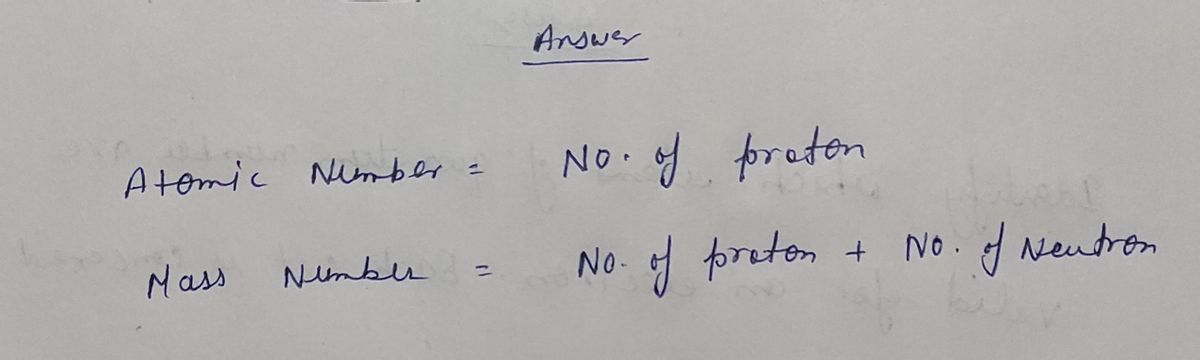
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY