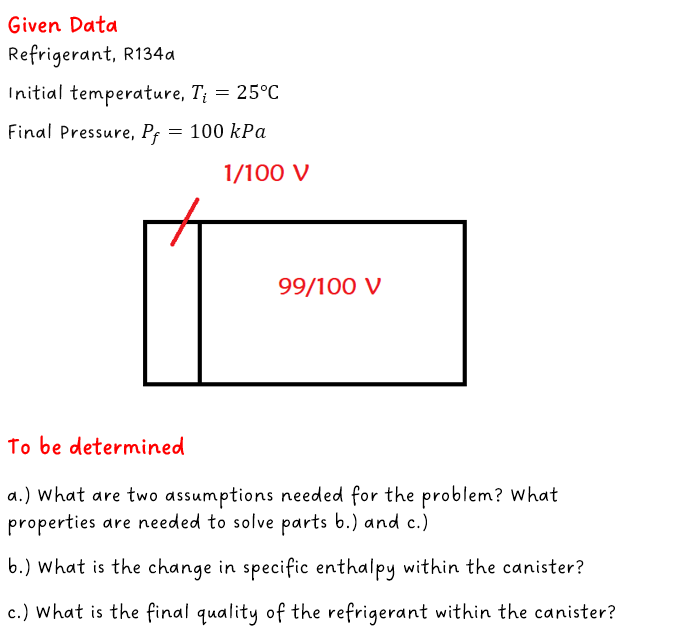
Refrigerant-134a is in an insulated canister with temperature 25 C. There is a partition in the canister so the refrigerant is a saturated liquid and takes up 1/100 of the volume of the canister. The rest of the volume is evacuated. When the canister is activated by squeezing (no work done activating) the partition breaks and the refrigerant fills the total volume. Final pressure is 100 kPa. a.) What are two assumptions needed for the problem? What properties are needed to solve part b.) and c.) b.) What is the change in specific enthalpy within the canister? c.) What is the final quality of the refrigerant within the canister?
Refrigerant-134a is in an insulated canister with temperature 25 C. There is a partition in the canister so the refrigerant is a saturated liquid and takes up 1/100 of the volume of the canister. The rest of the volume is evacuated. When the canister is activated by squeezing (no work done activating) the partition breaks and the refrigerant fills the total volume. Final pressure is 100 kPa. a.) What are two assumptions needed for the problem? What properties are needed to solve part b.) and c.) b.) What is the change in specific enthalpy within the canister? c.) What is the final quality of the refrigerant within the canister?
Refrigeration and Air Conditioning Technology (MindTap Course List)
8th Edition
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Chapter47: High-pressure, Low-pressure, And Absorption Chilled-water Systems
Section: Chapter Questions
Problem 18RQ: In an absorption chiller, _______is used to create the difference in pressures.
Related questions
Question
Refrigerant-134a is in an insulated canister with temperature 25 C. There is a partition in the canister so the refrigerant is a saturated liquid and takes up 1/100 of the volume of the canister. The rest of the volume is evacuated. When the canister is activated by squeezing (no work done activating) the partition breaks and the refrigerant fills the total volume. Final pressure is 100 kPa.
a.) What are two assumptions needed for the problem? What properties are needed to solve part b.) and c.)
b.) What is the change in specific enthalpy within the canister?
c.) What is the final quality of the refrigerant within the canister?
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning