reaction steps. A carbon tetrachloride production process consists of a reactor and a separator sys Agas stream containing 90 mole % chlorine and the balance methane is fed to the reactor. In the reactor a single-pass methane conversion of essentially 100% is attair the mole ratio of carbon tetrachloride to chloroform in the reactor product is 4:1, and negligible amounts of methyl chloride and methylene chloride are formed. The produca stream flows to a
reaction steps. A carbon tetrachloride production process consists of a reactor and a separator sys Agas stream containing 90 mole % chlorine and the balance methane is fed to the reactor. In the reactor a single-pass methane conversion of essentially 100% is attair the mole ratio of carbon tetrachloride to chloroform in the reactor product is 4:1, and negligible amounts of methyl chloride and methylene chloride are formed. The produca stream flows to a
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question

Transcribed Image Text:chlorination to form methylene chloride (CH₂Cl₂), chloroform (CHCl3), and carbon
tetrachloride (CC14) in different reaction steps.
A carbon tetrachloride production process consists of a reactor and a separator system.
gas
gas stream containing 90 mole % chlorine and the balance methane is fed to the
deactor. In the reactor a single-pass methane conversion of essentially 100% is attained,
the mole ratio of carbon tetrachloride to chloroform in the reactor product is 4:1, and
negligible amounts of methyl chloride and methylene chloride are formed. The product
stream flows to a separator, from which two streams emerge: a liquid, which contains
essentially all of the carbon tetrachloride and chloroform in the reactor effluent, and the
gas containing the chlorine and hydrogen chloride.
(a)
(b)
(c)
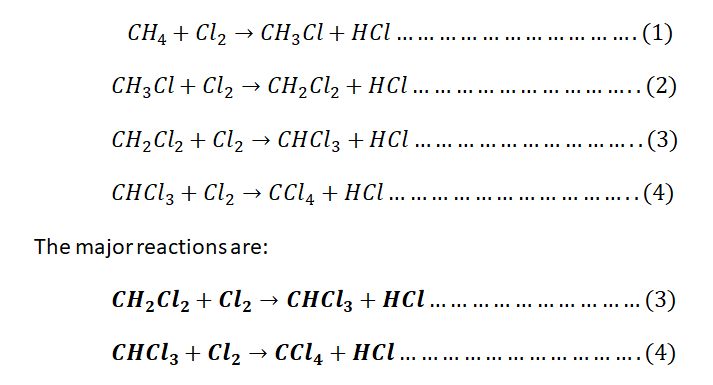
Write the chemical equations of the four reactions of methane chlorination and
give the two main overall reactions ignoring the ones producing negligible
amounts.
Explain the reason for the chlorine presence in the reactor outlet stream.
Using a basis of 100 kmol.h¹ combined feed to the reactor, calculate the molar
flow rate and molar composition of the reactor outlet.
14

Transcribed Image Text:Problem 3
Methane reacts with chlorine (atomic weight 35.5) to produce methyl chloride (CH3CI)
and hydrogen chloride (HCI). Once formed, the methyl chloride may undergo further
chlorination to form methylene chloride (CH₂C₂), chloroform (CHCb), and carbon
tetrachloride (CCl4) in different reaction steps.
A carbon tetrachloride production process consists of a reactor and a separator system.
A gas stream containing 90 mole % chlorine and the balance methane is fed to the
reactor. In the reactor a single-pass methane conversion of essentially 100% is attained,
the mole ratio of carbon tetrachloride to chloroform in the reactor product is 4:1, and
negligible amounts of methyl chloride and methylene chloride are formed. The product
stream flows to a separator, from which two streams emerge: a liquid, which contains
essentially all of the carbon tetrachloride and chloroform in the reactor effluent, and the
gas containing the chlorine and hydrogen chloride.
the four reactions of mathana chlorination and
14
Expert Solution
Step 1: (a)The 4 chemical reactions of chlorination of methane and the 2 major overall reactions
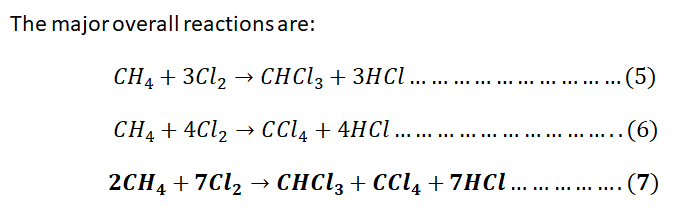
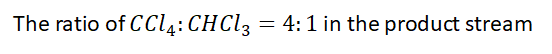
Step by step
Solved in 4 steps with 10 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The